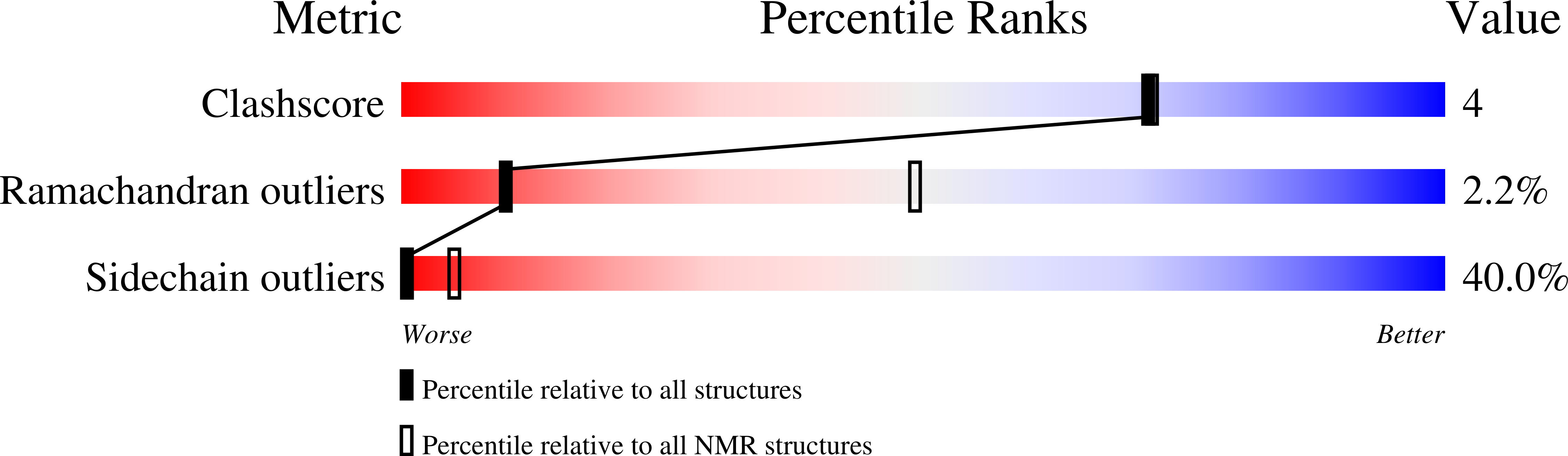Deposition Date
1996-03-16
Release Date
1996-12-07
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1OEG
Keywords:
Title:
PEPTIDE OF HUMAN APOE RESIDUES 267-289, NMR, 5 STRUCTURES AT PH 6.0, 37 DEGREES CELSIUS AND PEPTIDE:SDS MOLE RATIO OF 1:90
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
Conformers Submitted:
5