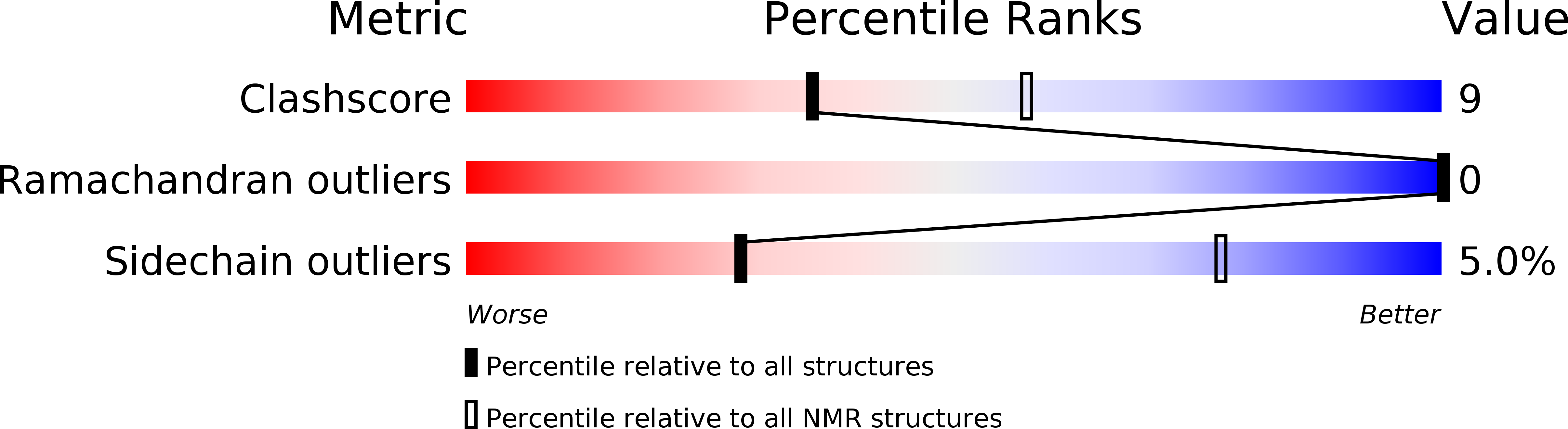Deposition Date
2002-12-09
Release Date
2003-03-13
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1O8Y
Keywords:
Title:
Solution structure of SFTI-1(6,5), an acyclic permutant of the proteinase inhibitor SFTI-1, trans-trans-trans conformer (tt-A)
Biological Source:
Source Organism:
HELIANTHUS ANNUUS L. (Taxon ID: 4232)
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
LEAST OVERALL ENERGIES