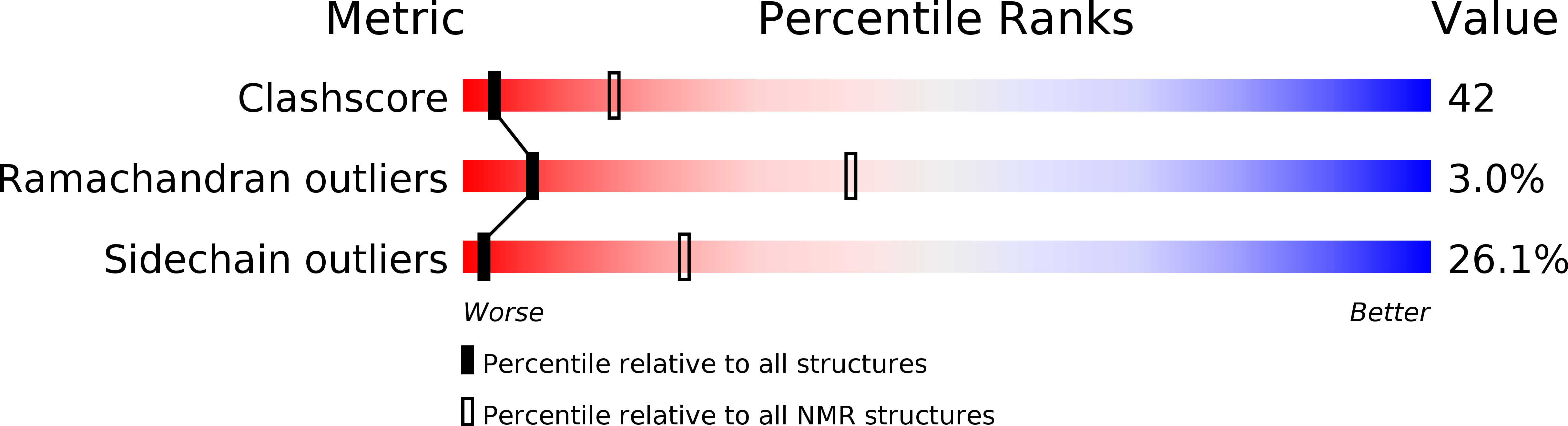
Deposition Date
2002-10-17
Release Date
2003-01-30
Last Version Date
2024-05-15
Entry Detail
PDB ID:
1O6X
Keywords:
Title:
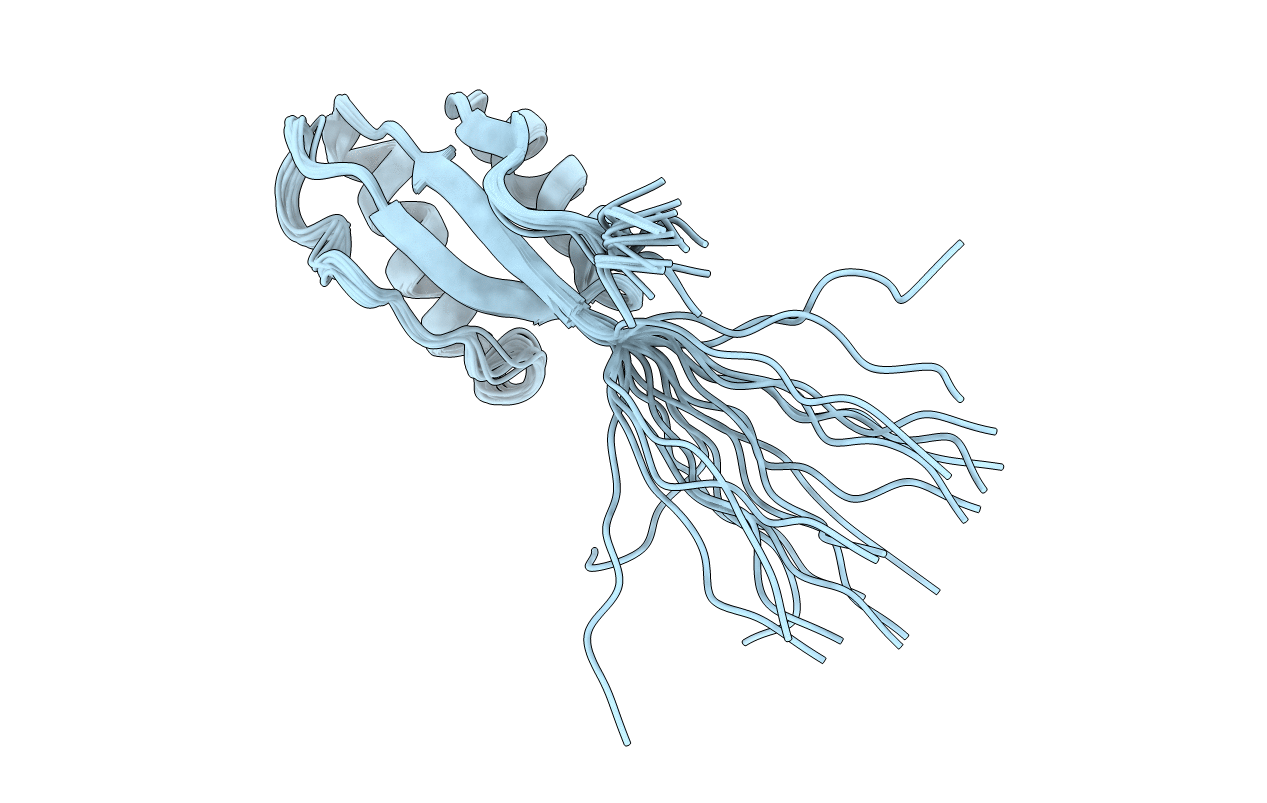
NMR solution structure of the activation domain of human procarboxypeptidase A2
Biological Source:
Source Organism:
HOMO SAPIENS (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Conformers Submitted:
20
Selection Criteria:
LEAST RESTRAINT VIOLATION