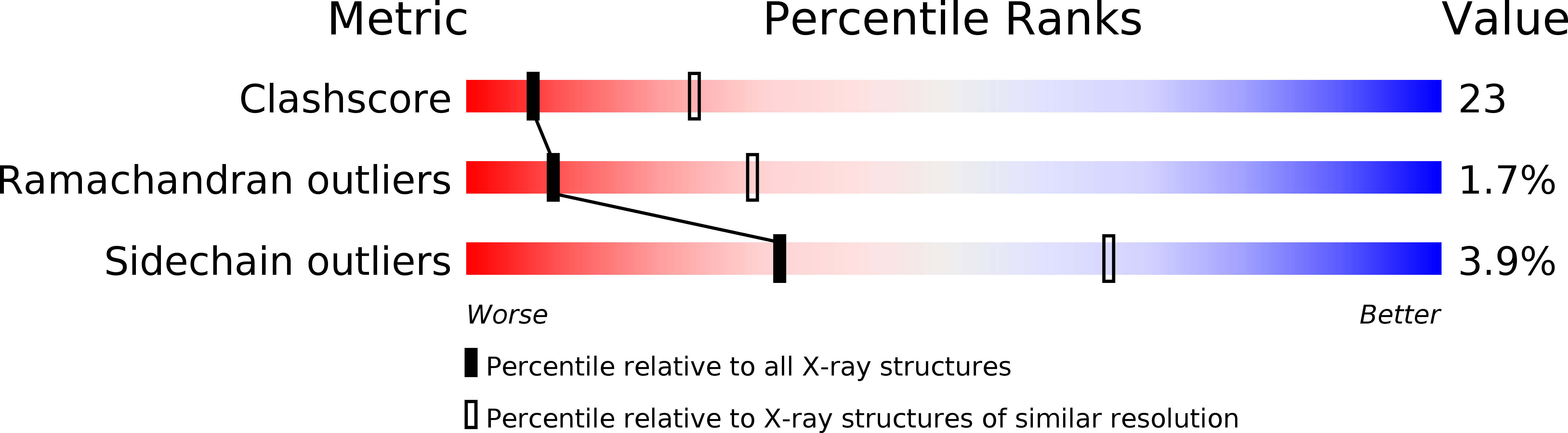
Deposition Date
2002-10-10
Release Date
2003-10-17
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1O6O
Keywords:
Title:
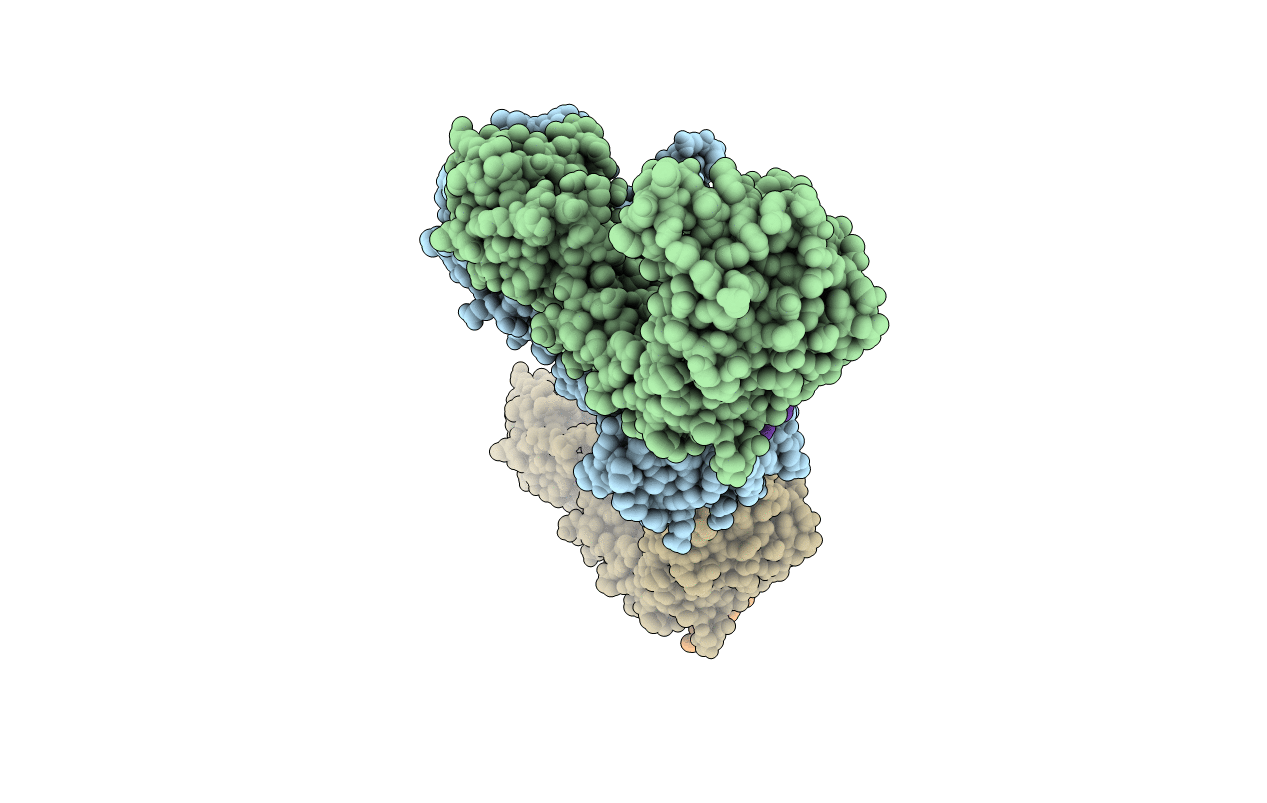
Importin Beta aa1-442 bound to five FxFG repeats from yeast Nsp1p. Second crystal form
Biological Source:
Source Organism:
HOMO SAPIENS (Taxon ID: 9606)
SACCHAROMYCES CEREVISIAE (Taxon ID: 4932)
SACCHAROMYCES CEREVISIAE (Taxon ID: 4932)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.80 Å
R-Value Free:
0.27
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 21 21 21