Deposition Date
2003-04-14
Release Date
2003-07-15
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1O3W
Keywords:
Title:
Structure of the inhibitor free triple mutant (K53,56,120M) of phospholipase A2
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
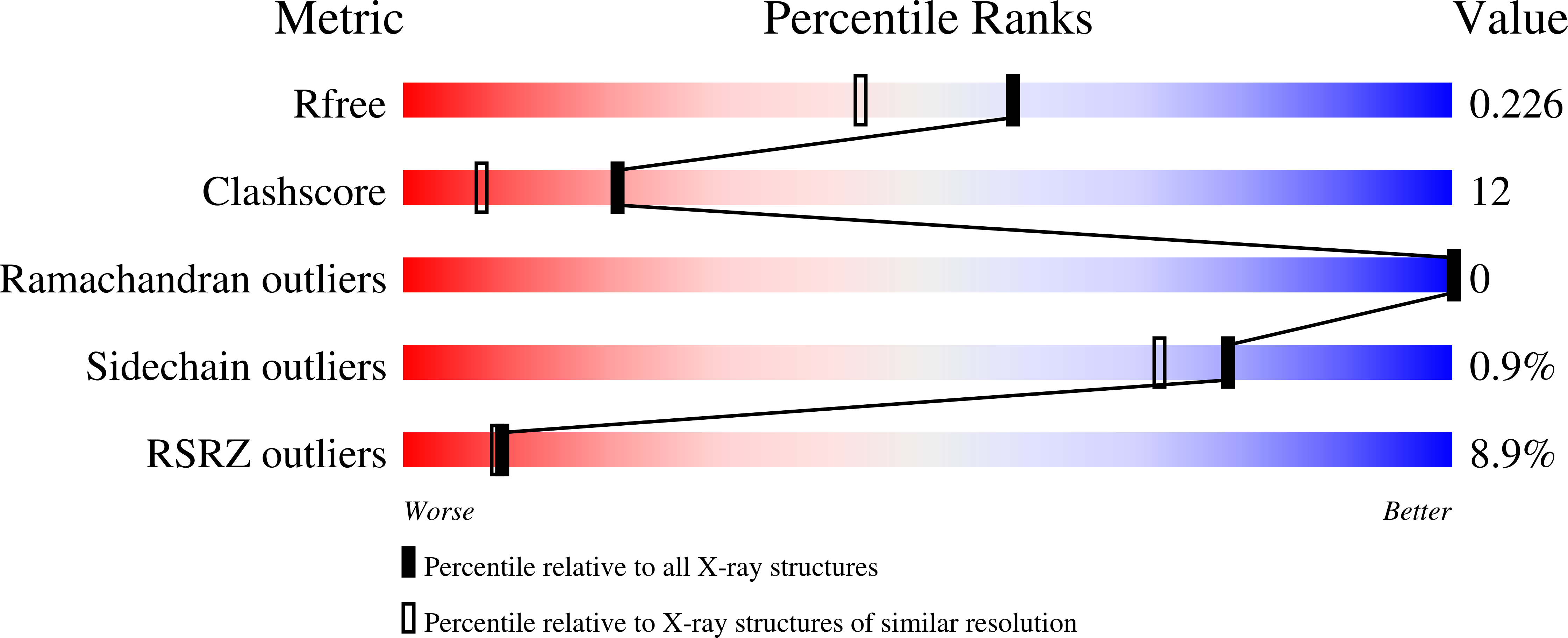
Resolution:
1.85 Å
R-Value Free:
0.23
R-Value Work:
0.19
Space Group:
P 31 2 1