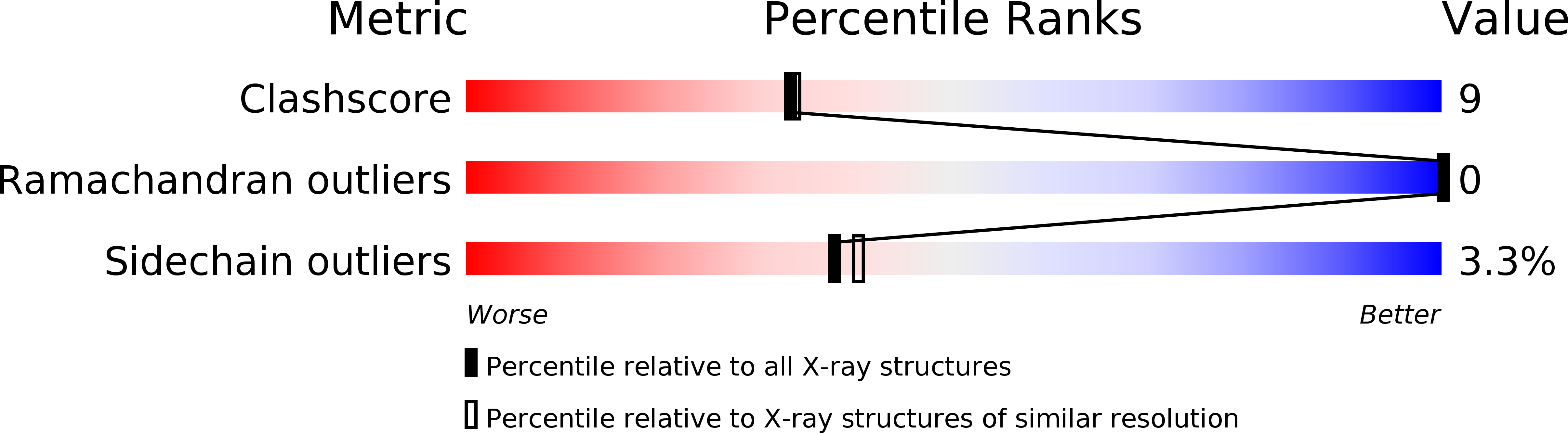
Deposition Date
1995-04-18
Release Date
1995-07-10
Last Version Date
2024-06-05
Entry Detail
PDB ID:
1NSP
Keywords:
Title:
MECHANISM OF PHOSPHATE TRANSFER BY NUCLEOSIDE DIPHOSPHATE KINASE: X-RAY STRUCTURES OF A PHOSPHO-HISTIDINE INTERMEDIATE OF THE ENZYMES FROM DROSOPHILA AND DICTYOSTELIUM
Biological Source:
Source Organism(s):
Dictyostelium discoideum (Taxon ID: 44689)
Method Details:
Experimental Method:
Resolution:
2.10 Å
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 63 2 2