Deposition Date
2003-01-21
Release Date
2003-04-22
Last Version Date
2023-08-16
Entry Detail
PDB ID:
1NQA
Keywords:
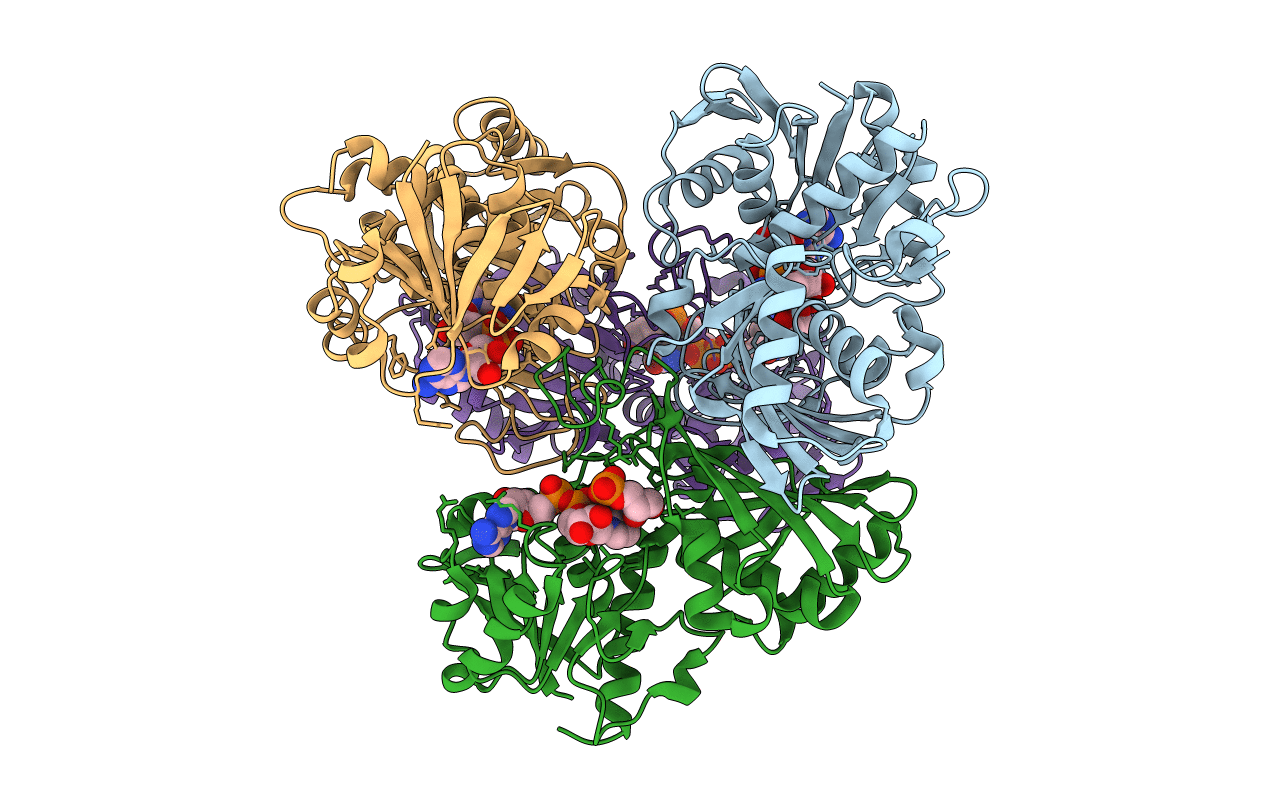
Title:
Glyceraldehyde-3-Phosphate Dehydrogenase Mutant With Cys 149 Replaced By Ala Complexed With Nad+ and D-Glyceraldehyde-3-Phosphate
Biological Source:
Source Organism:
Geobacillus stearothermophilus (Taxon ID: 1422)
Host Organism:
Method Details:
Experimental Method:
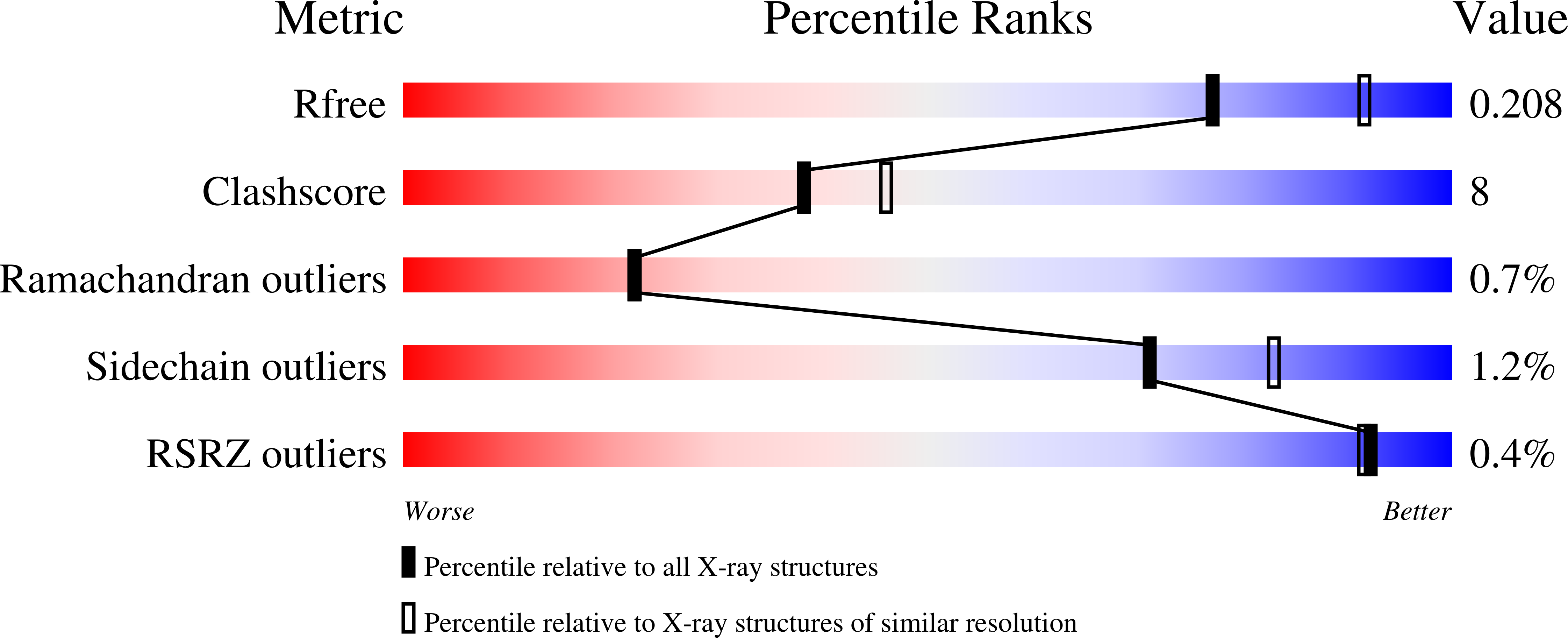
Resolution:
2.20 Å
R-Value Free:
0.21
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 31 2 1