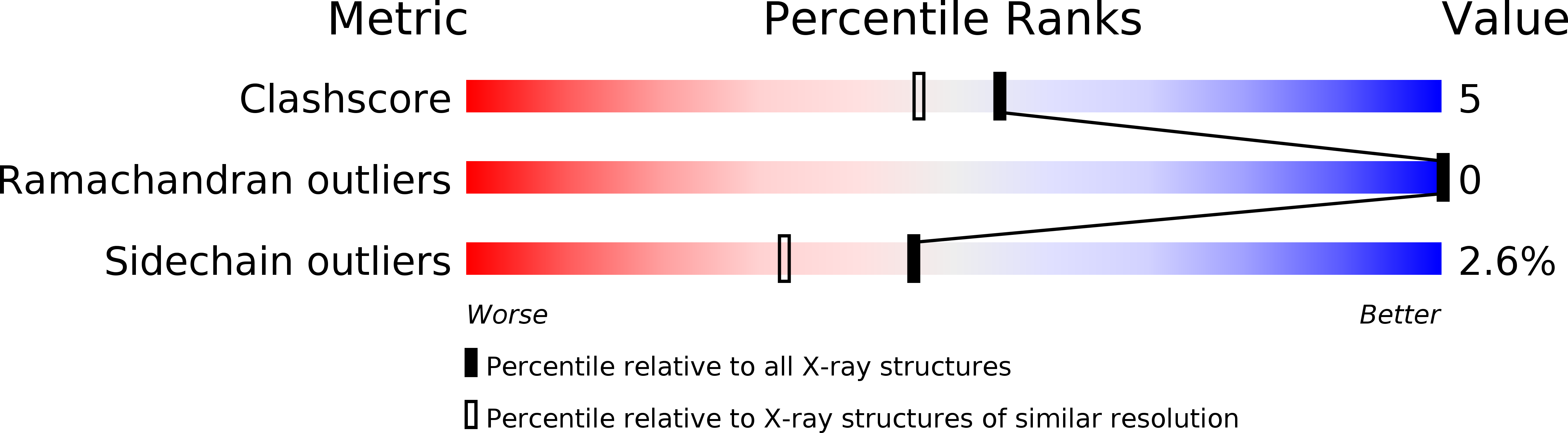Deposition Date
1995-03-15
Release Date
1996-04-03
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1NNC
Keywords:
Title:
INFLUENZA VIRUS NEURAMINIDASE SUBTYPE N9 (TERN) COMPLEXED WITH 4-GUANIDINO-NEU5AC2EN INHIBITOR
Biological Source:
Source Organism(s):
Method Details:
Experimental Method:
Resolution:
1.80 Å
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
I 4 3 2