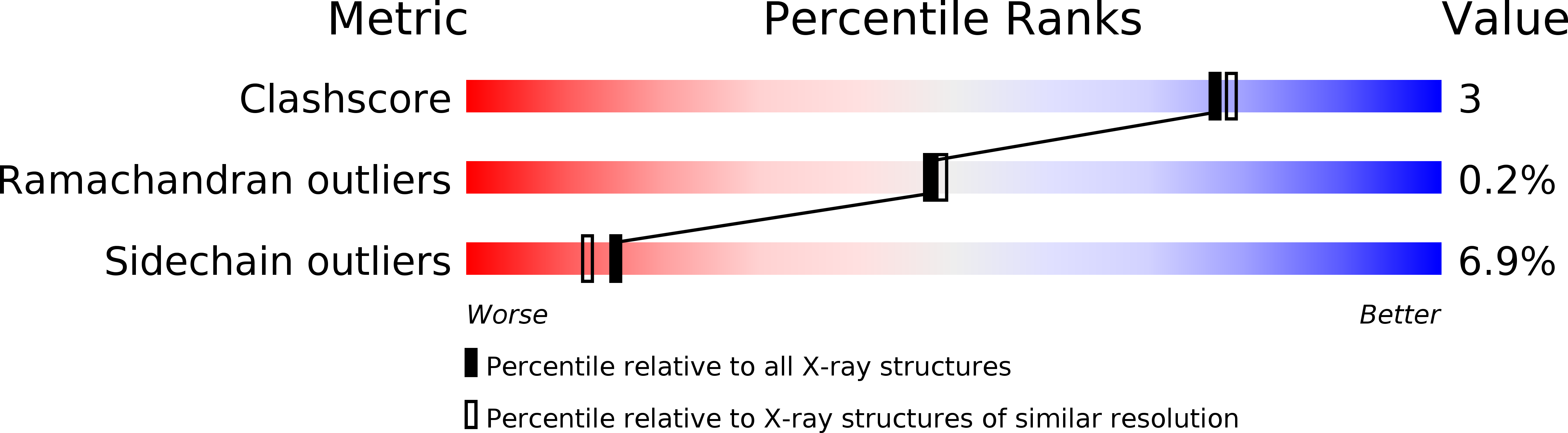Deposition Date
1994-12-09
Release Date
1995-02-14
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1NHR
Keywords:
Title:
AN L40C MUTATION CONVERTS THE CYSTEINE-SULFENIC ACID REDOX CENTRE IN ENTEROCOCCAL NADH PEROXIDASE TO A DISULFIDE
Biological Source:
Source Organism(s):
Enterococcus faecalis (Taxon ID: 1351)
Method Details:
Experimental Method:
Resolution:
2.10 Å
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
I 2 2 2