Abstact
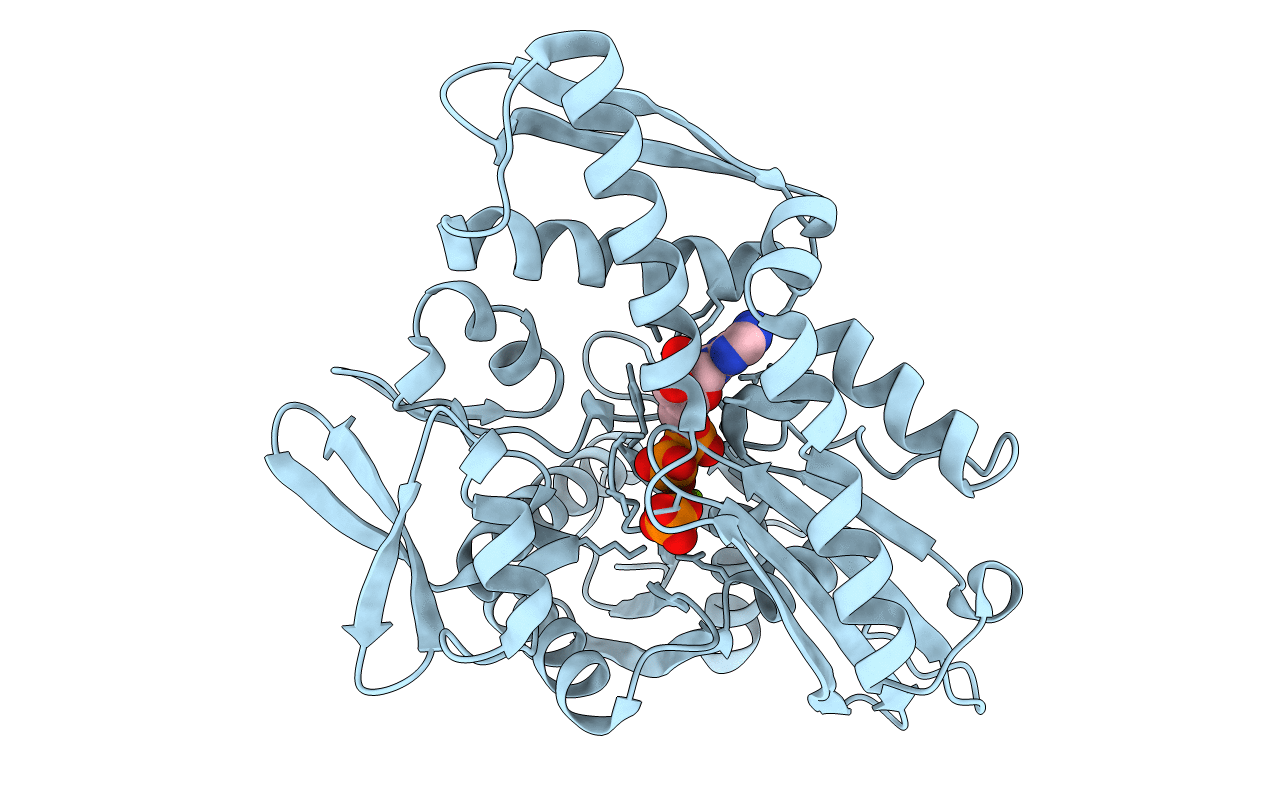
The ATPase fragment of the bovine 70-kDa heat shock cognate protein is an attractive construct in which to study its mechanism of ATP hydrolysis. The three-dimensional structure suggests several residues that might participate in the ATPase reaction. Four acidic residues (Asp-10, Glu-175, Asp-199, and Asp-206) have been individually mutated to both the cognate amine (asparagine/glutamine) and to serine, and the effects of the mutations on the kinetics of the ATPase activity (Wilbanks, S. M., DeLuca-Flaherty, C., and McKay, D. B. (1994) J. Biol. Chem. 269, 12893-12898) and the structure of the mutant ATPase fragments have been determined, typically to approximately 2.4 A resolution. Additionally, the structures of the wild type protein complexed with MgADP and Pi, MgAMPPNP (5'-adenylyl-beta, gamma-imidodiphosphate) and CaAMPPNP have been refined to 2.1, 2.4, and 2.4 A, respectively. Combined, these structures provide models for the prehydrolysis, MgATP-bound state and the post-hydrolysis, MgADP-bound state of the ATPase fragment. These models suggest a pathway for the hydrolytic reaction in which 1) the gamma phosphate of bound ATP reorients to form a beta, gamma-bidentate phosphate complex with the Mg2+ ion, allowing 2) in-line nucleophilic attack on the gamma phosphate by a H2O molecule or OH- ion, with 3) subsequent release of inorganic phosphate.