Deposition Date
2002-11-27
Release Date
2003-11-18
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1NAK
Keywords:
Title:
IGG1 FAB FRAGMENT (83.1) COMPLEX WITH 16-RESIDUE PEPTIDE (RESIDUES 304-321 OF HIV-1 GP120 (MN ISOLATE))
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Method Details:
Experimental Method:
Resolution:
2.57 Å
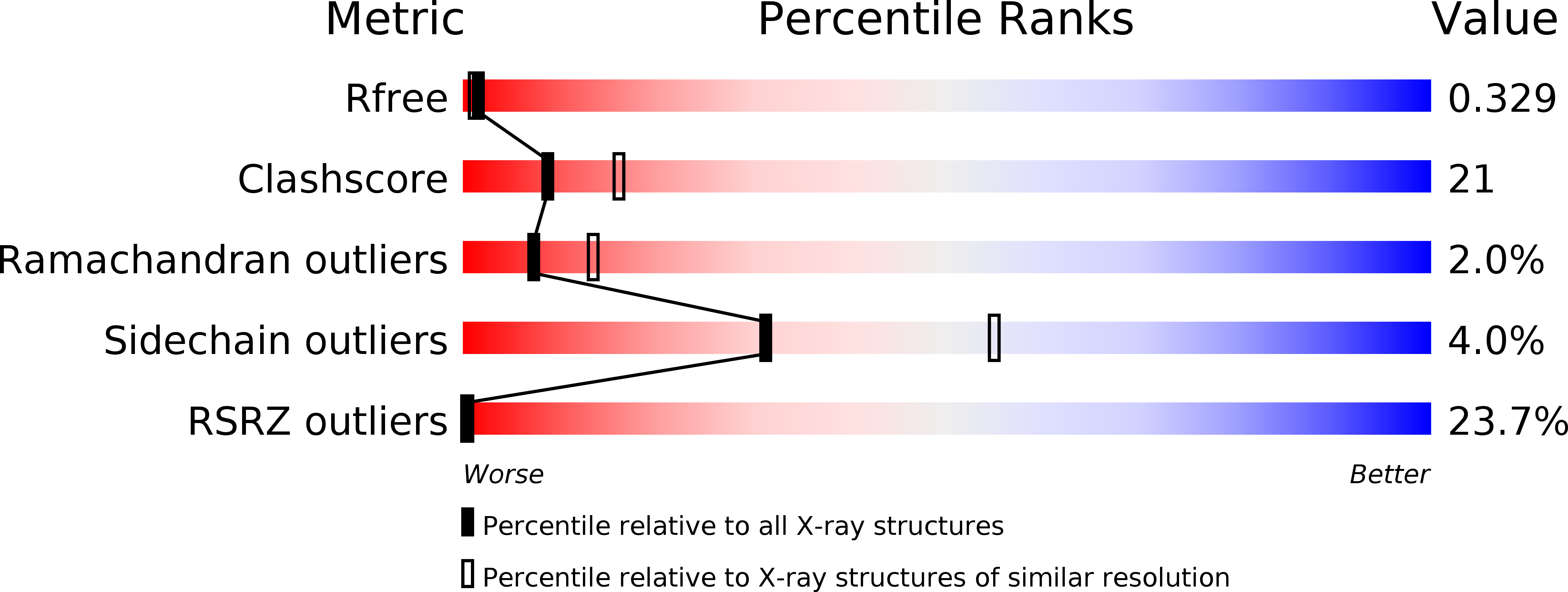
R-Value Free:
0.32
R-Value Work:
0.28
Space Group:
P 1 21 1