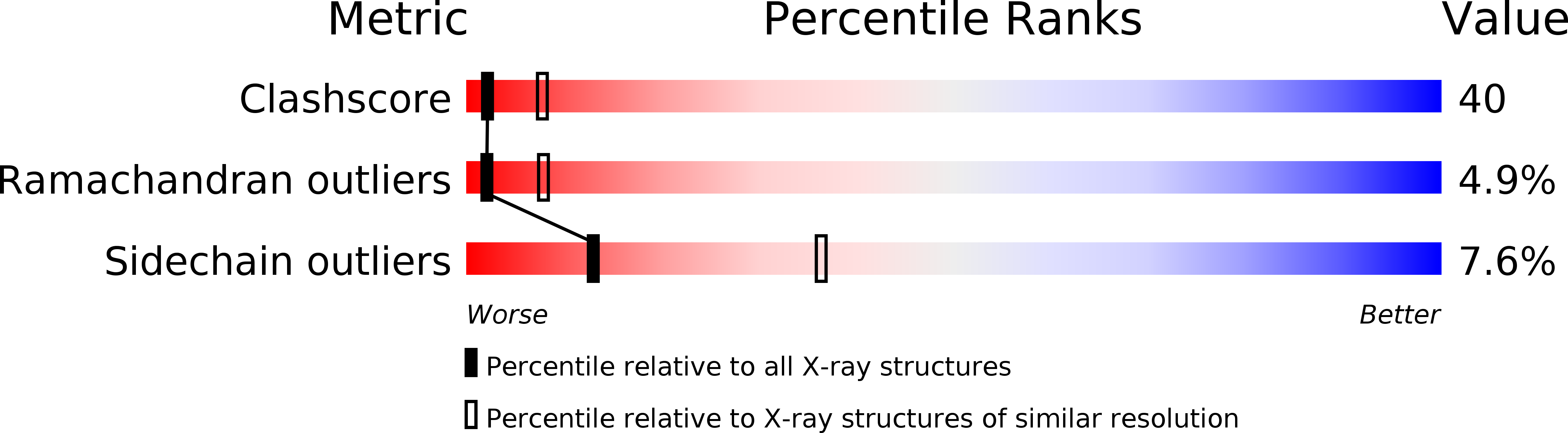Deposition Date
2002-11-12
Release Date
2003-01-07
Last Version Date
2024-12-25
Entry Detail
PDB ID:
1N73
Keywords:
Title:
Fibrin D-Dimer, Lamprey complexed with the PEPTIDE LIGAND: GLY-HIS-ARG-PRO-AMIDE
Biological Source:
Source Organism(s):
Petromyzon marinus (Taxon ID: 7757)
Method Details:
Experimental Method:
Resolution:
2.90 Å
R-Value Free:
0.28
R-Value Work:
0.25
R-Value Observed:
0.26
Space Group:
P 1