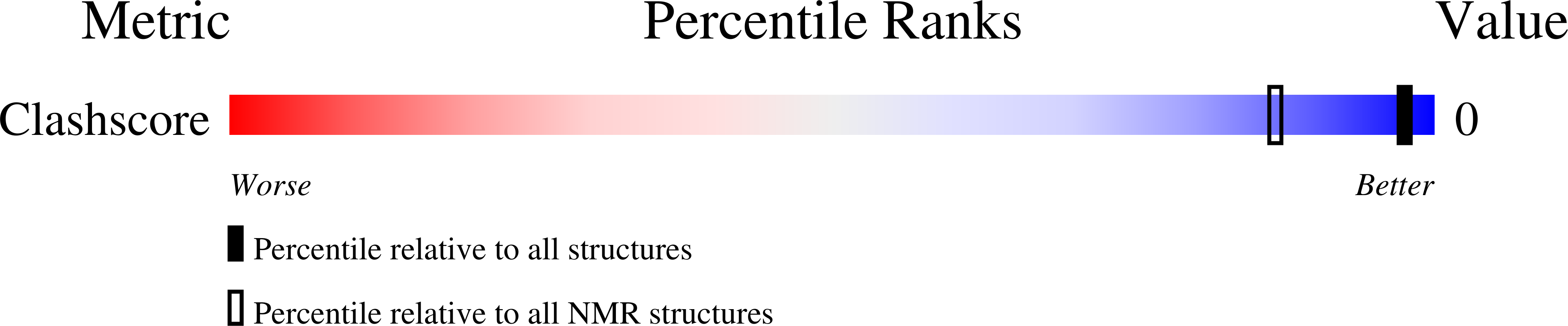
Deposition Date
2002-10-16
Release Date
2002-10-23
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1N14
Keywords:
Title:
Structure and Dynamics of Thioguanine-modified Duplex DNA in Comparison with Unmodified DNA; Structure of Unmodified Duplex DNA
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
20
Selection Criteria:
back calculated data agree with experimental NOESY spectrum