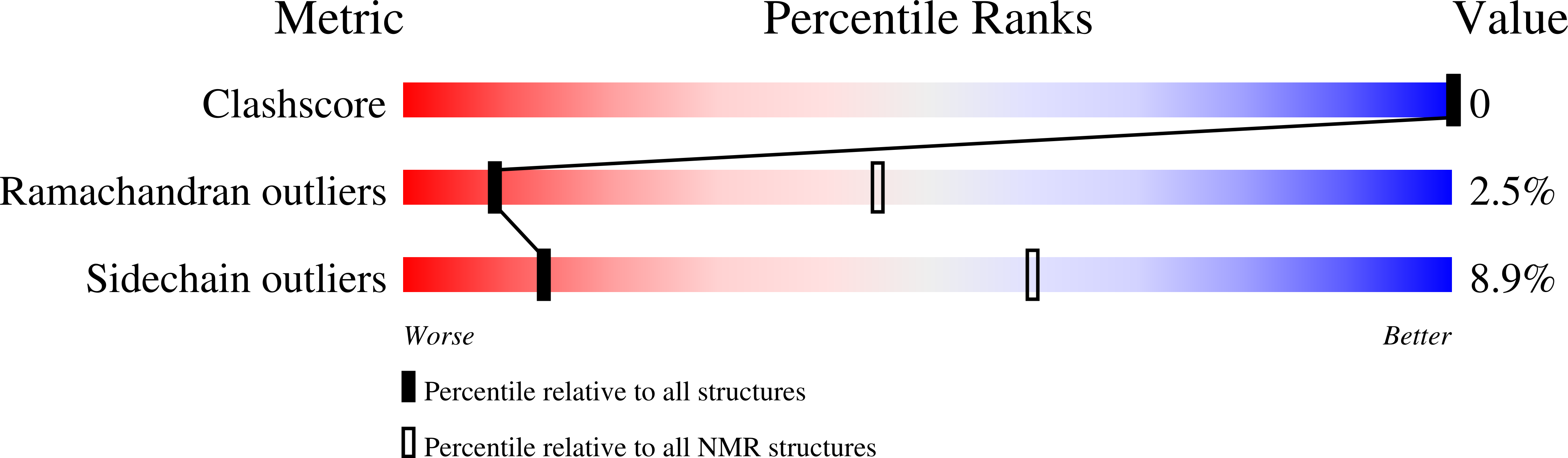
Deposition Date
2002-10-11
Release Date
2003-02-04
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1N0D
Keywords:
Title:
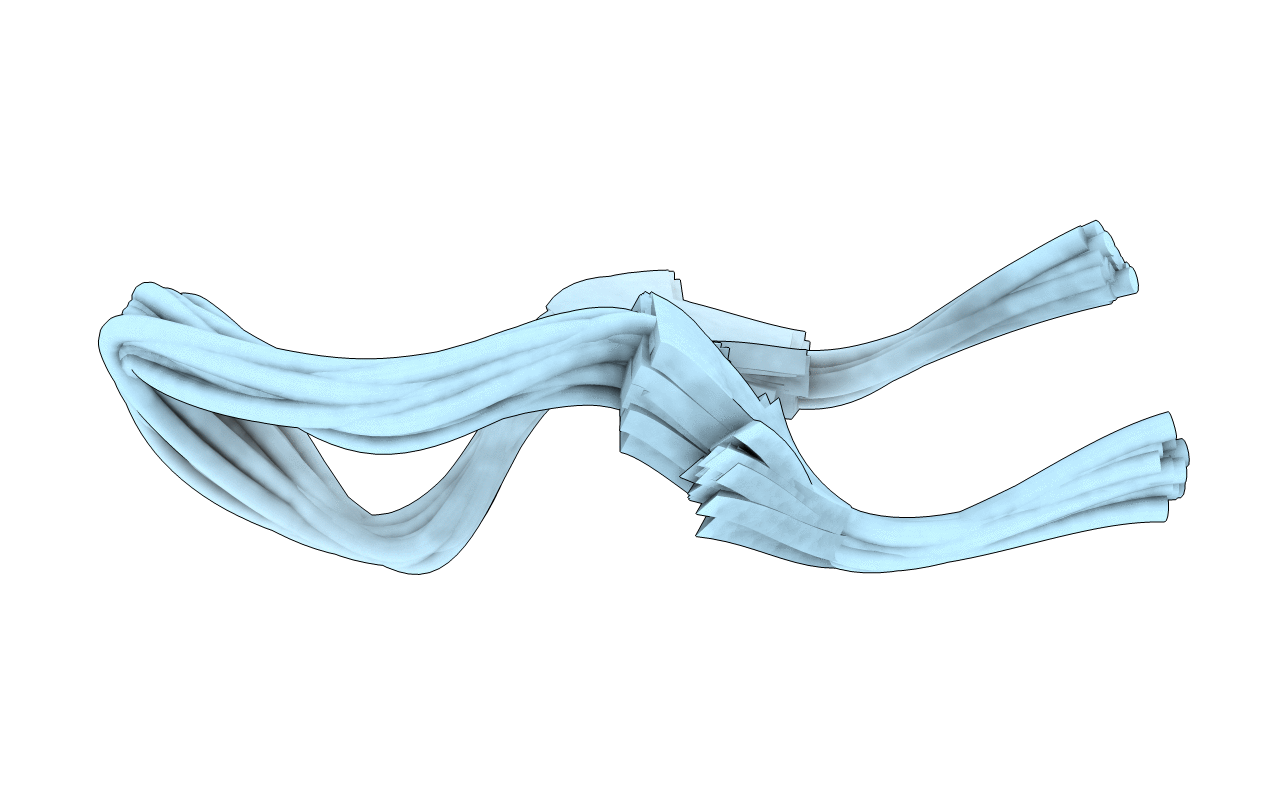
Stability of cyclic beta-hairpins: Asymmetric contibutions from side chains of hydrogen bonded cross-strand residue pair
Method Details:
Experimental Method:
Conformers Calculated:
80
Conformers Submitted:
20
Selection Criteria:
structures with the least restraint violations