Abstact
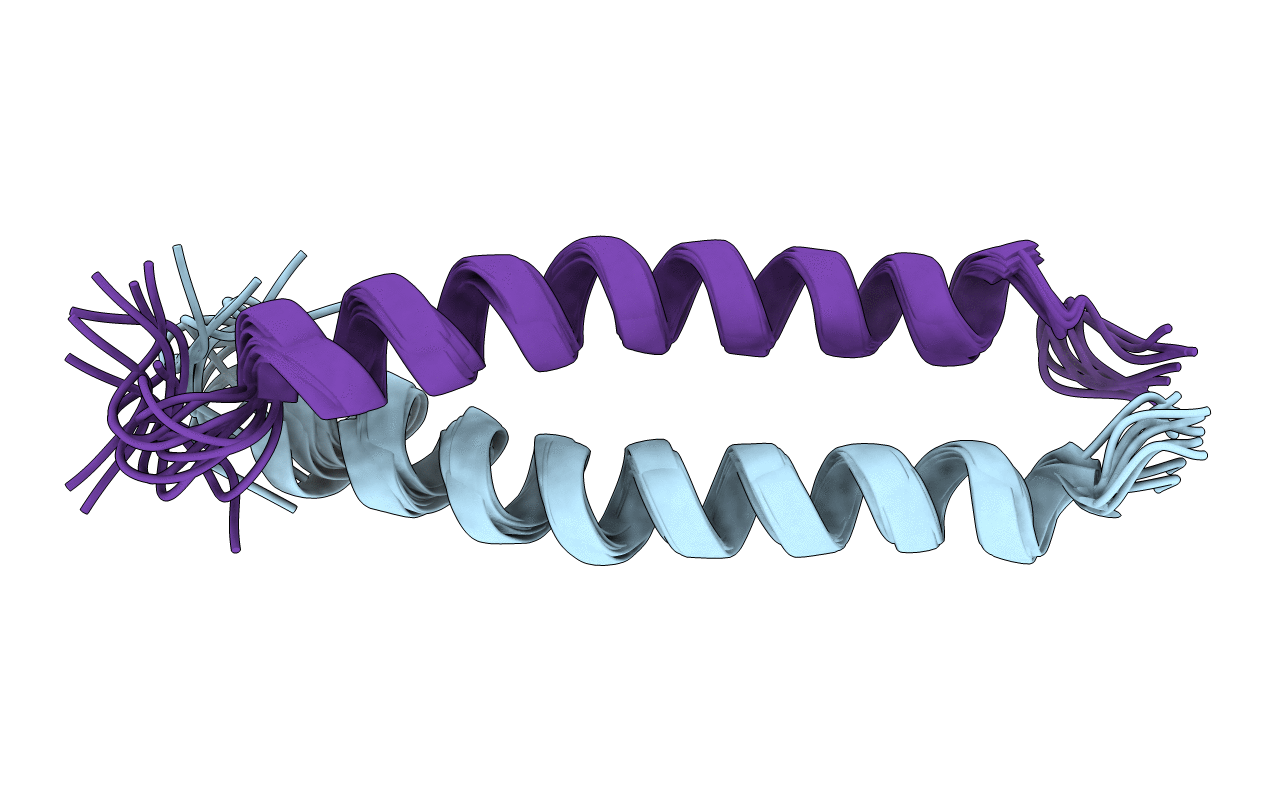
Coiled coils are well-known as oligomerization domains, but they are also important sites of protein-protein interactions. We determined the NMR solution structure and backbone (15)N relaxation rates of a disulfide cross-linked, two-chain, 37-residue polypeptide containing the 34 C-terminal residues of striated muscle alpha-tropomyosin, TM9a(251-284). The peptide binds to the N-terminal region of TM and to the tropomyosin-binding domain of the regulatory protein, troponin T. Comparison of the NMR solution structure of TM9a(251-284) with the X-ray structure of a related peptide [Li, Y., Mui, S., Brown, J. H., Strand, J., Reshetnikova, L., Tobacman, L. S., and Cohen, C. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 7378-7383] reveals significant differences. In solution, residues 253-269 (like most of the tropomyosin molecule) form a canonical coiled coil. Residues 270-279, however, are parallel, linear helices, novel for tropomyosin. The packing between the parallel helices results from unusual interface residues that are atypical for coiled coils. Y267 has poor packing at the coiled-coil interface and a lower R(2) relaxation rate than neighboring residues, suggesting there is conformational flexibility around this residue. The last five residues are nonhelical and flexible. The exposed surface presented by the parallel helices, and the flexibility around Y267 and the ends, may facilitate binding to troponin T and formation of complexes with the N-terminus of tropomyosin and actin. We propose that unusual packing and flexibility are general features of coiled-coil domains in proteins that are involved in intermolecular interactions.