Abstact
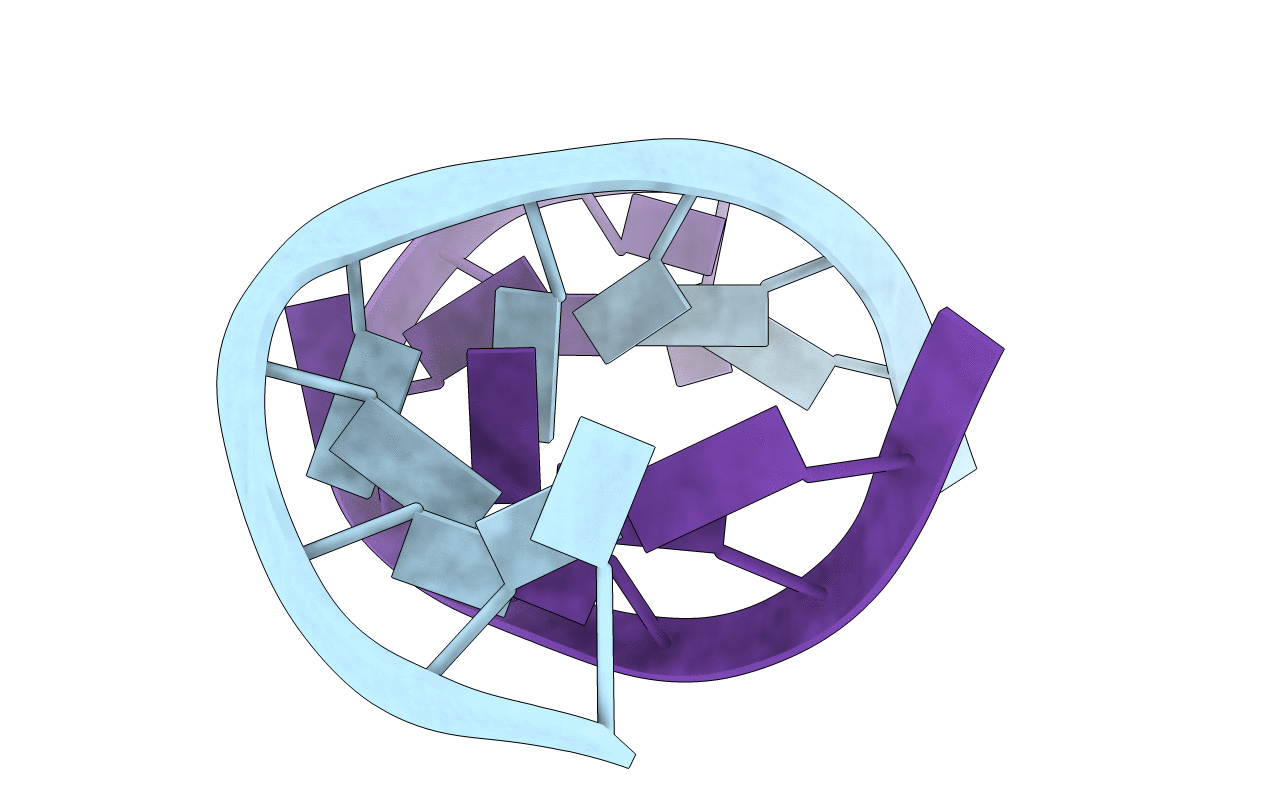
The contribution of amino groups to the thermodynamics, structure, and dynamics of tandem A.A mismatches is investigated by substitution of purine (P) for adenine (A) within the RNA duplex, 5'(rGGCAAGCCU)(2), to give 5'(rGGCPAGCCU)(2), 5'(rGGCAPGCCU)(2), and 5'(rGGCPPGCCU)(2). The 5'(rGGCAAGCCU)(2) duplex has sheared A(anti).A(anti) (A.A trans Hoogsteen/Sugar-edge) pairs in which the A5 amino group is involved in hydrogen bonds but the A4 amino group is not [Znosko, B. M., Burkard, M. E., Schroeder, S. J., Krugh, T. R., and Turner, D. H. (2002) Biochemistry 41, 14969-14977]. In comparison to 5'(rGGCAAGCCU)(2), replacing the amino group of A4 with a hydrogen stabilizes the duplex by 1.3 kcal/mol, replacement of the A5 amino group destabilizes the duplex by 0.6 kcal/mol, and replacement of both A4 and A5 amino groups destabilizes the duplex by 0.8 kcal/mol. In NMR structures, the P.A noncanonical pairs of the 5'(rGGCPAGCCU)(2) duplex have a sheared anti-anti structure (P.A trans Hoogsteen/Sugar-edge) with P4.A5 interstrand hydrogen bonding and A5 bases that interstrand stack, similar to the structure of 5'(rGGCAAGCCU)(2). In contrast, the A.P pairs of the 5'(rGGCAPGCCU)(2) duplex have a face-to-face conformation (A.P trans Watson-Crick/Watson-Crick) with intrastrand stacking resembling typical A-form geometry. Although the P5 bases in 5'(rGGCPPGCCU)(2) are involved in an interstrand stack, the loop region is largely undefined. The results illustrate that both hydrogen-bonded and non-hydrogen-bonded amino groups play important roles in determining the thermodynamic, structural, and dynamic characteristics of purine rich internal loops.