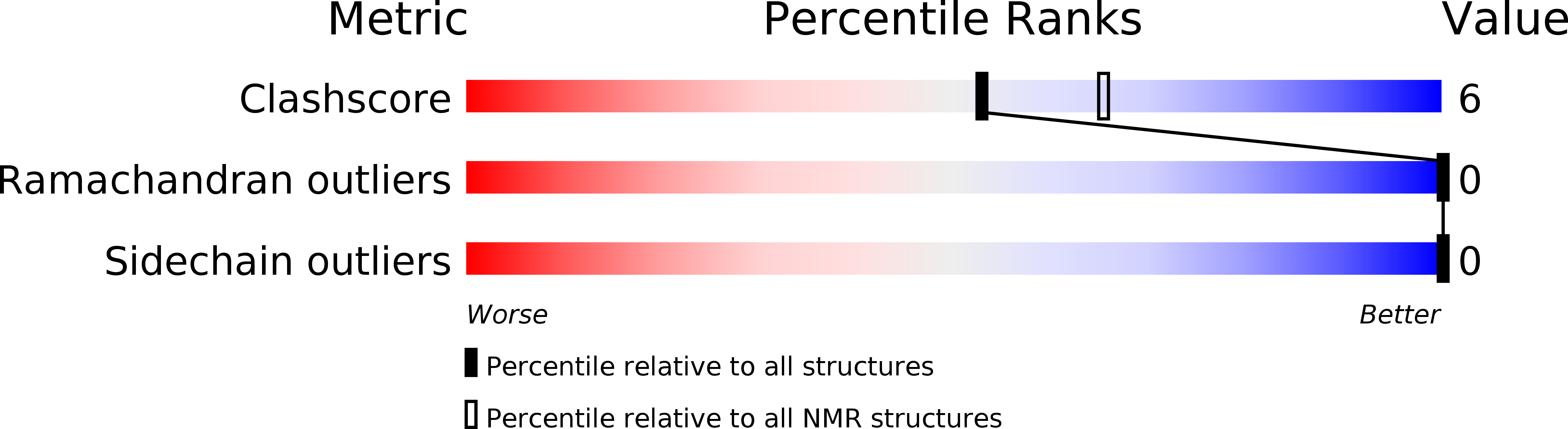
Deposition Date
2002-09-22
Release Date
2003-02-11
Last Version Date
2025-03-26
Entry Detail
PDB ID:
1MTQ
Keywords:
Title:
THREE-DIMENSIONAL SOLUTION STRUCTURE OF ALPHA-CONOTOXIN GID BY NMR SPECTROSCOPY
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
all calculated structures submitted, structures with the lowest energy