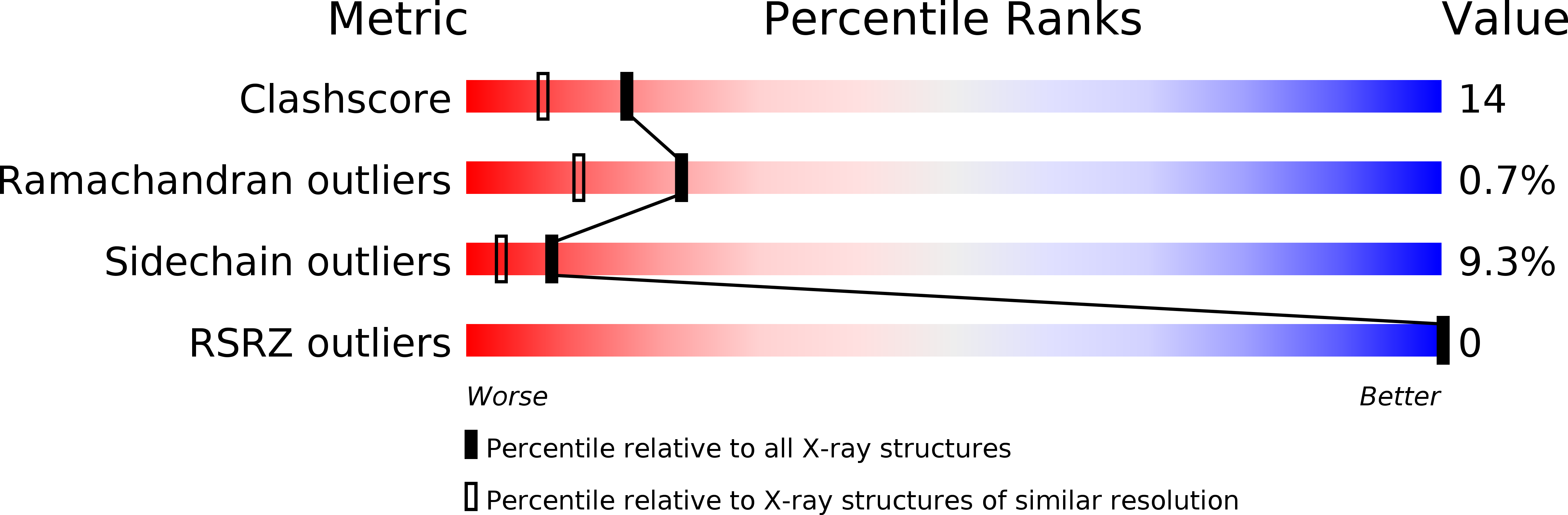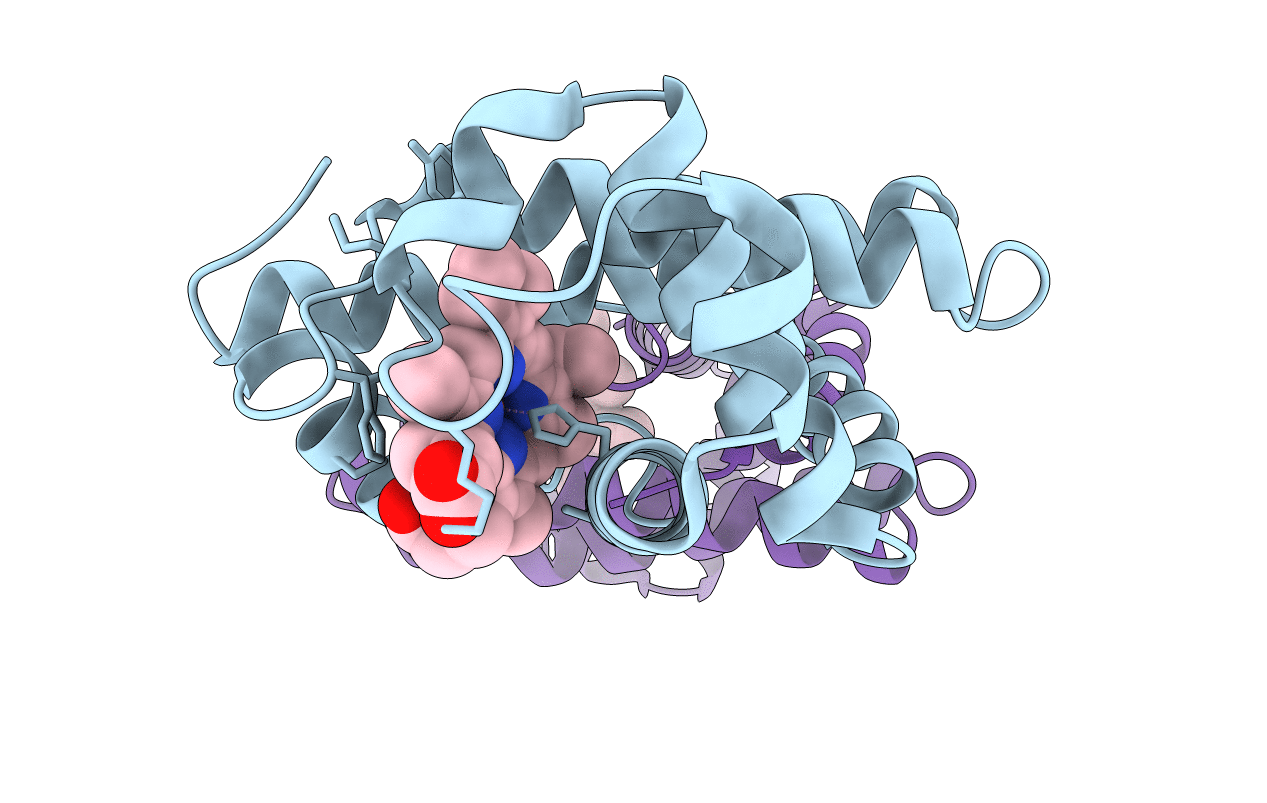
Deposition Date
1995-01-11
Release Date
1995-04-20
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1MNI
Keywords:
Title:
ALTERATION OF AXIAL COORDINATION BY PROTEIN ENGINEERING IN MYOGLOBIN. BIS-IMIDAZOLE LIGATION IN THE HIS64-->VAL(SLASH)VAL68-->HIS DOUBLE MUTANT
Biological Source:
Source Organism(s):
Sus scrofa (Taxon ID: 9823)
Method Details:
Experimental Method:
Resolution:
2.07 Å
R-Value Observed:
0.17
Space Group:
I 1 21 1