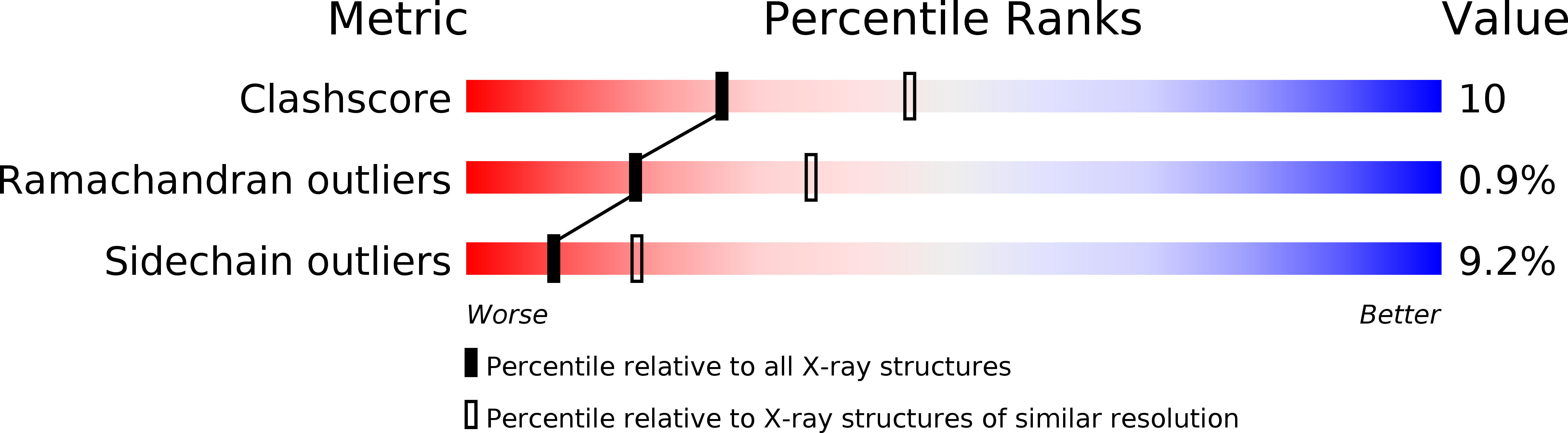
Deposition Date
1994-12-08
Release Date
1995-06-03
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1MHT
Keywords:
Title:
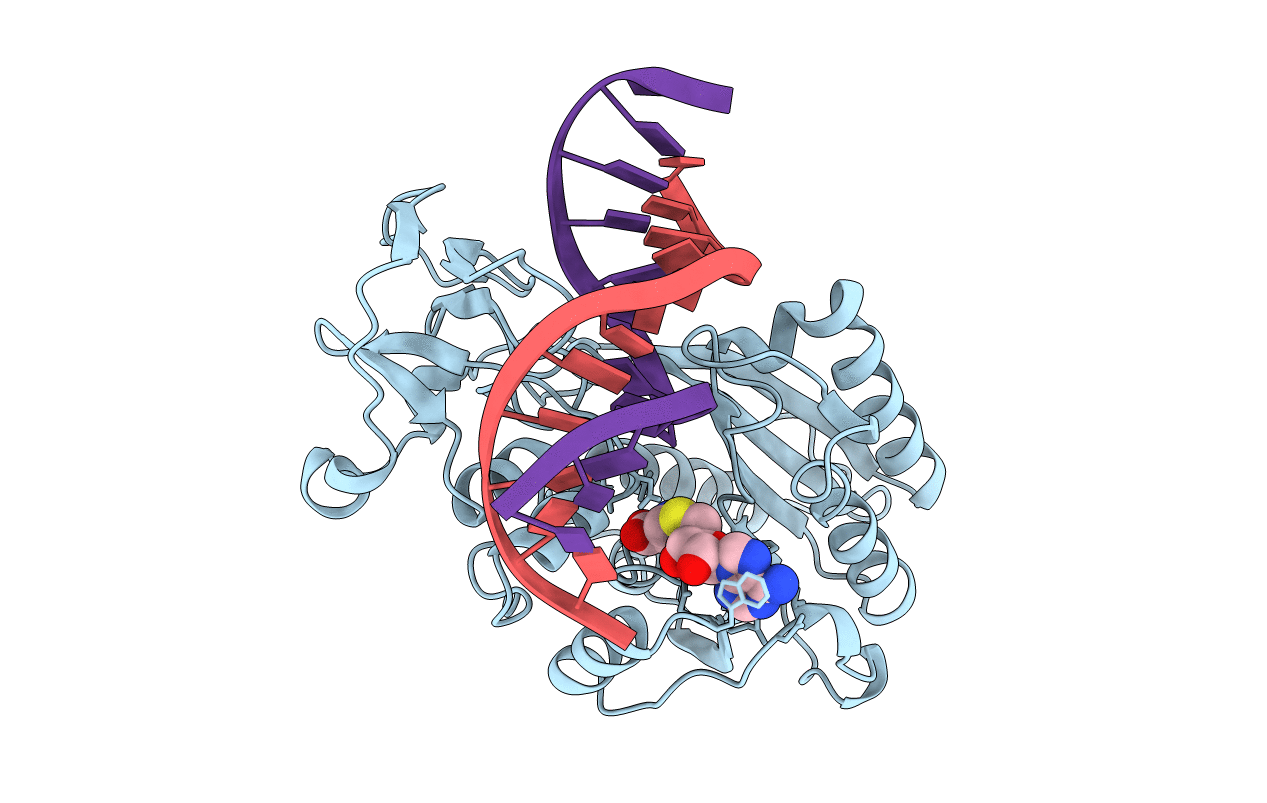
COVALENT TERNARY STRUCTURE OF HHAI METHYLTRANSFERASE, DNA AND S-ADENOSYL-L-HOMOCYSTEINE
Biological Source:
Source Organism(s):
Haemophilus haemolyticus (Taxon ID: 726)
Method Details:
Experimental Method:
Resolution:
2.60 Å
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
H 3 2