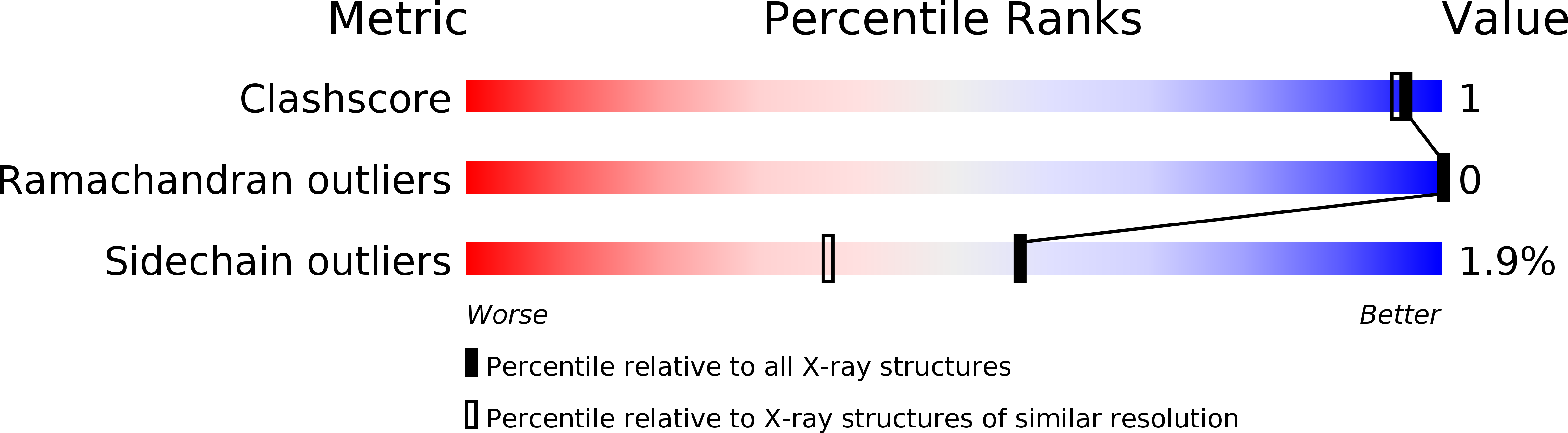
Deposition Date
1992-07-20
Release Date
1994-01-31
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1MDC
Keywords:
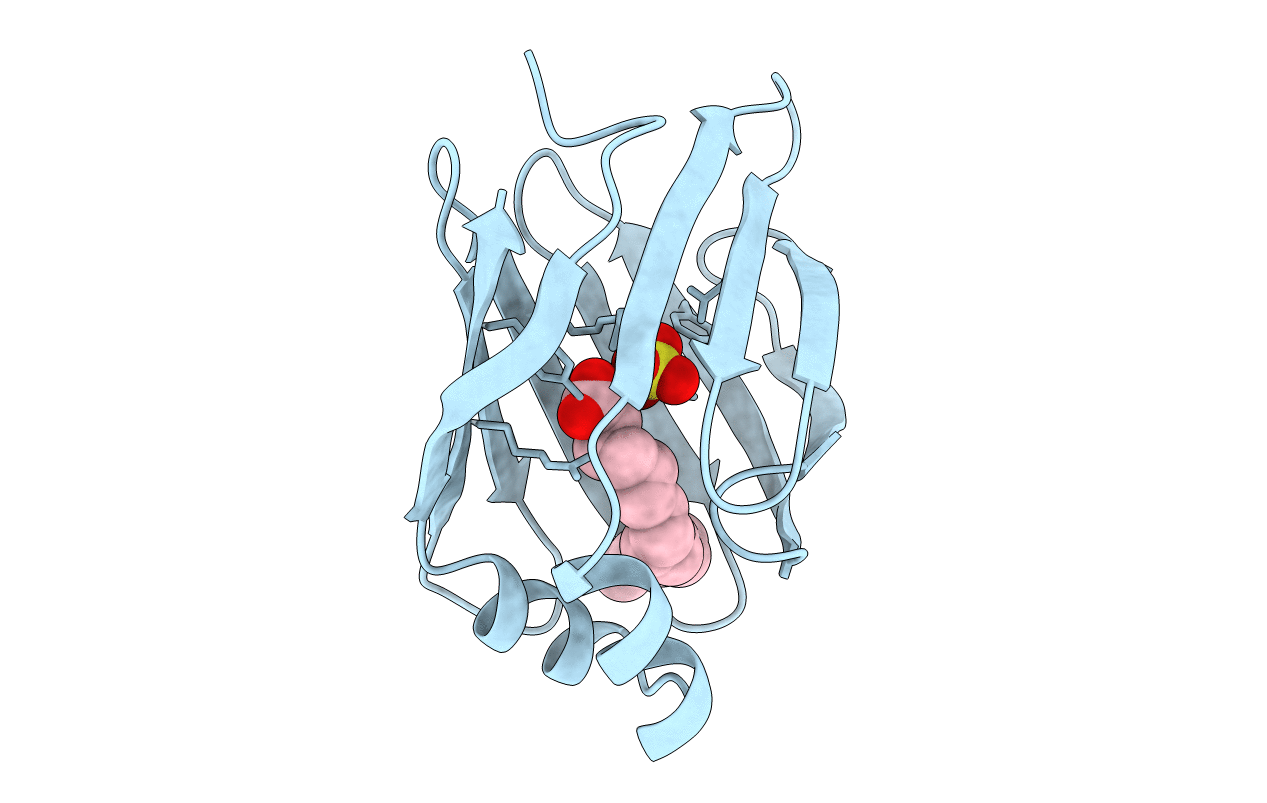
Title:
CRYSTALLIZATION, STRUCTURE DETERMINATION AND LEAST-SQUARES REFINEMENT TO 1.75 ANGSTROMS RESOLUTION OF THE FATTY-ACID-BINDING PROTEIN ISOLATED FROM MANDUCA SEXTA L
Biological Source:
Source Organism(s):
Manduca sexta (Taxon ID: 7130)
Method Details:
Experimental Method:
Resolution:
1.75 Å
R-Value Observed:
0.17
Space Group:
P 1 21 1