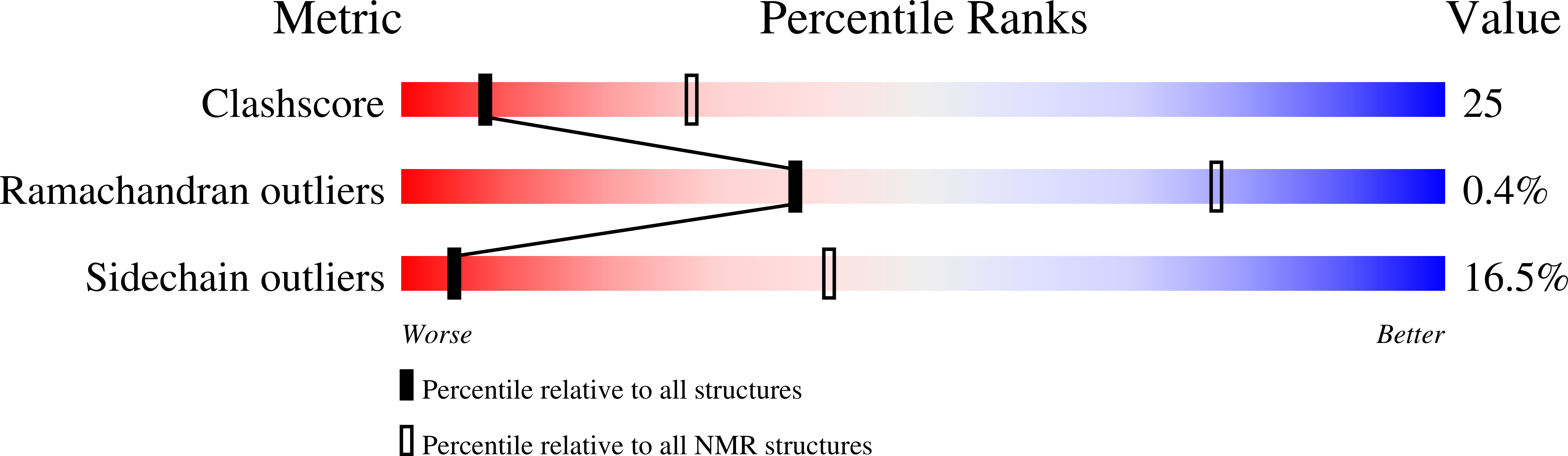
Deposition Date
2002-07-22
Release Date
2002-09-25
Last Version Date
2024-10-09
Entry Detail
PDB ID:
1M7T
Keywords:
Title:
Solution Structure and Dynamics of the Human-Escherichia coli Thioredoxin Chimera: Insights into Thermodynamic Stability
Biological Source:
Source Organism(s):
Homo sapiens, Escherichia coli (Taxon ID: 9606,562)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
21
Selection Criteria:
Back calculated data agree with experimental NOESY spectrum,structures with acceptable covalent geometry,structures with favorable non-bond energy,structures with the least restraint violations,structures with the lowest energy,target function