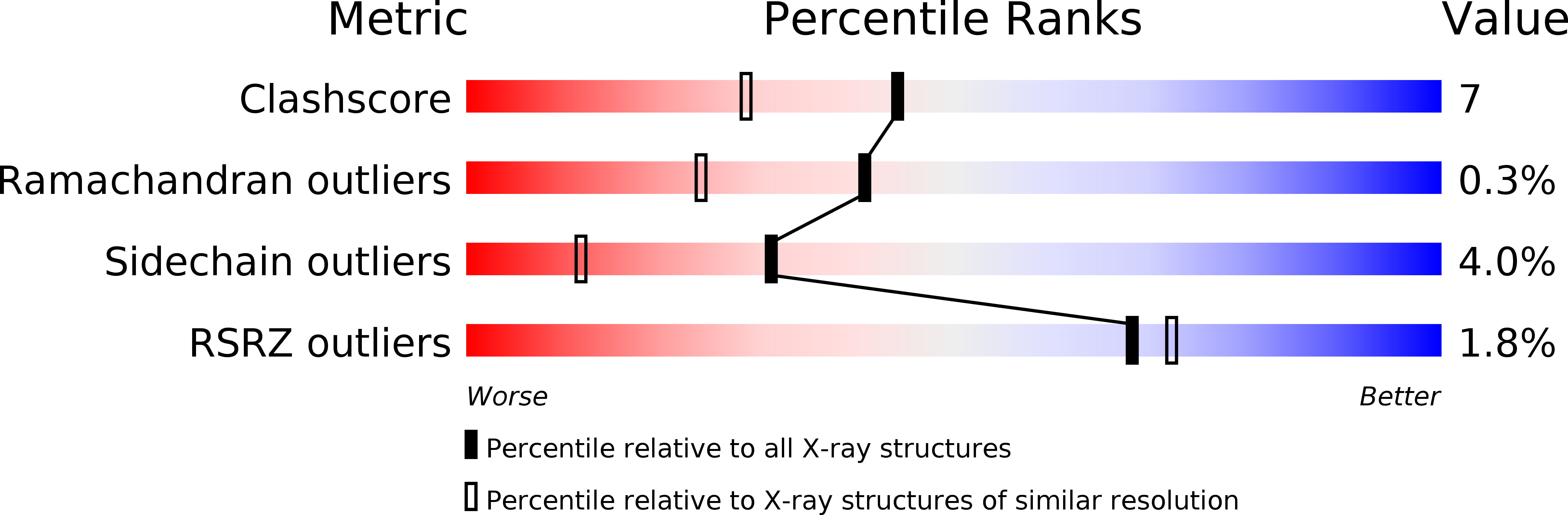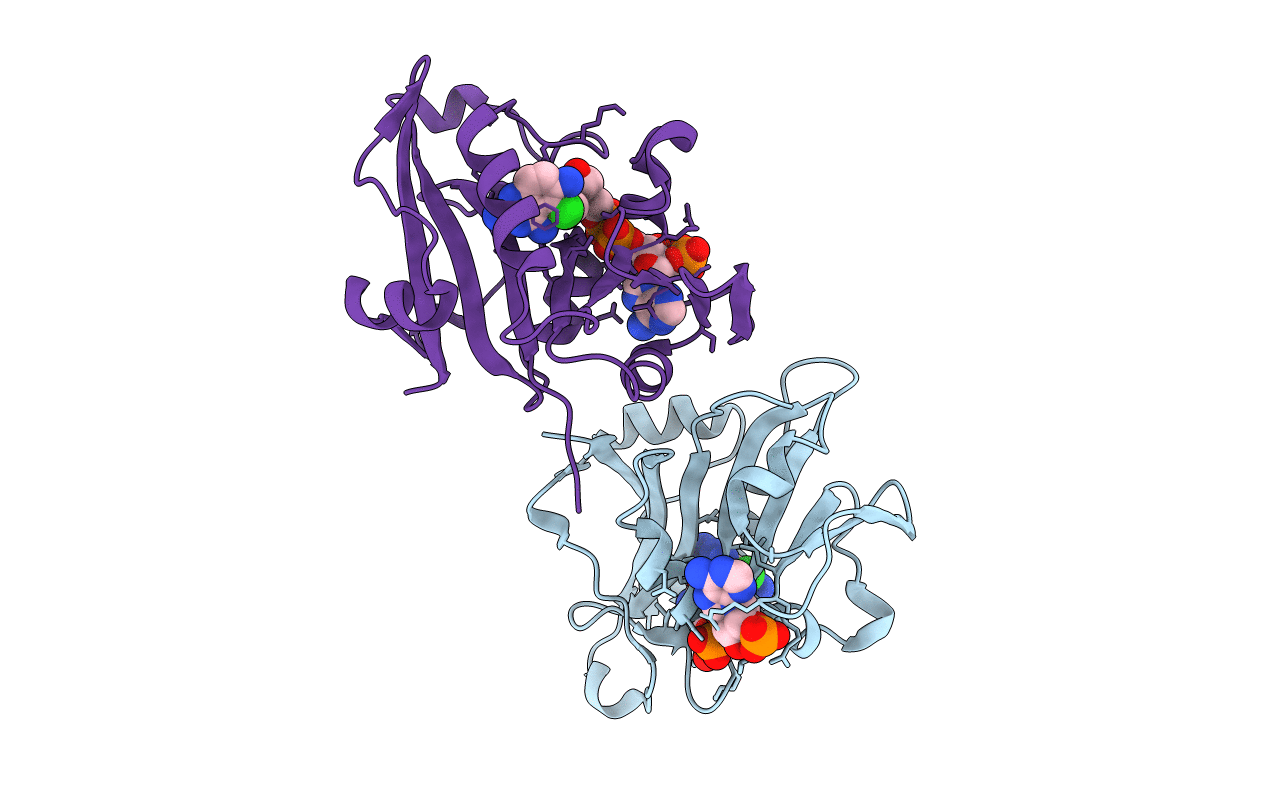
Deposition Date
2002-07-19
Release Date
2003-03-04
Last Version Date
2023-08-23
Entry Detail
PDB ID:
1M78
Keywords:
Title:
CANDIDA ALBICANS DIHYDROFOLATE REDUCTASE COMPLEXED WITH DIHYDRO-NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE (NADPH) AND 5-CHLORYL-2,4,6-QUINAZOLINETRIAMINE (GW1225)
Biological Source:
Source Organism(s):
Candida albicans (Taxon ID: 5476)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.71 Å
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 1 21 1