Deposition Date
2002-07-10
Release Date
2002-11-08
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1M5Y
Keywords:
Title:
Crystallographic Structure of SurA, a Molecular Chaperone that Facilitates Outer Membrane Porin Folding
Biological Source:
Source Organism:
Escherichia coli (Taxon ID: 562)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.00 Å
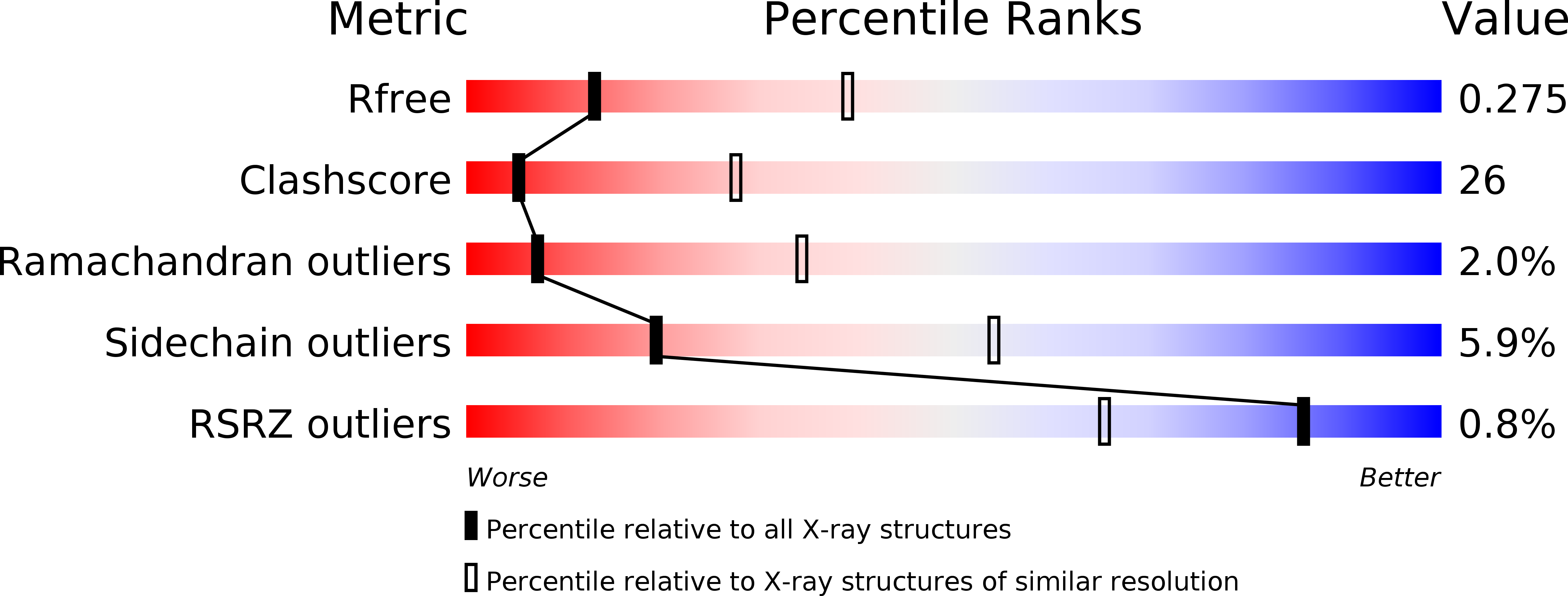
R-Value Free:
0.28
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
F 2 2 2