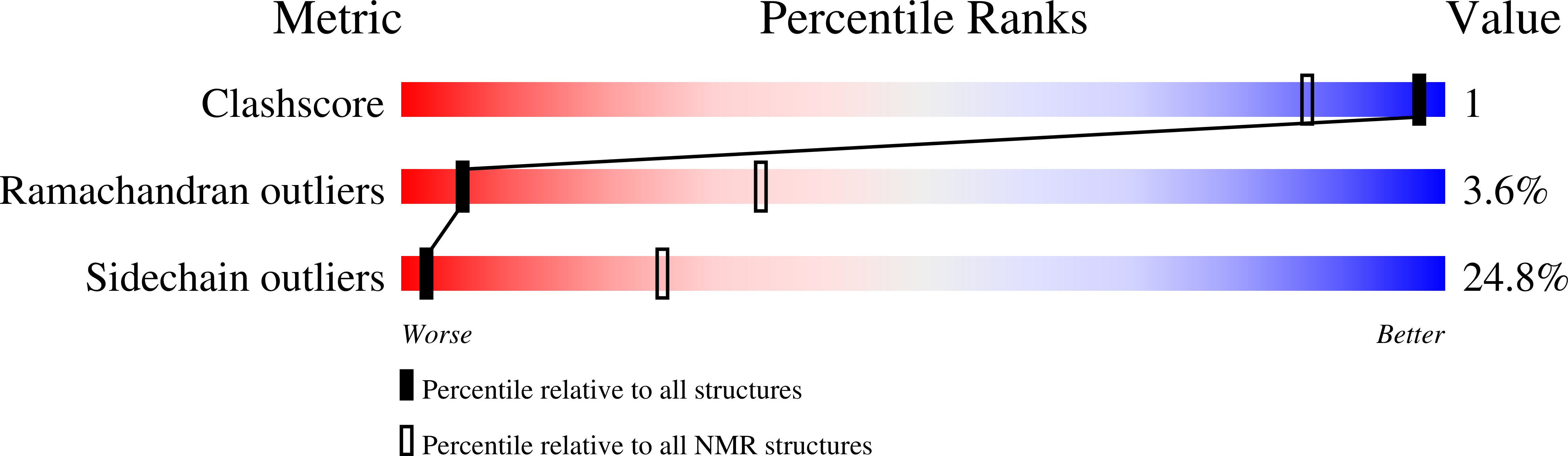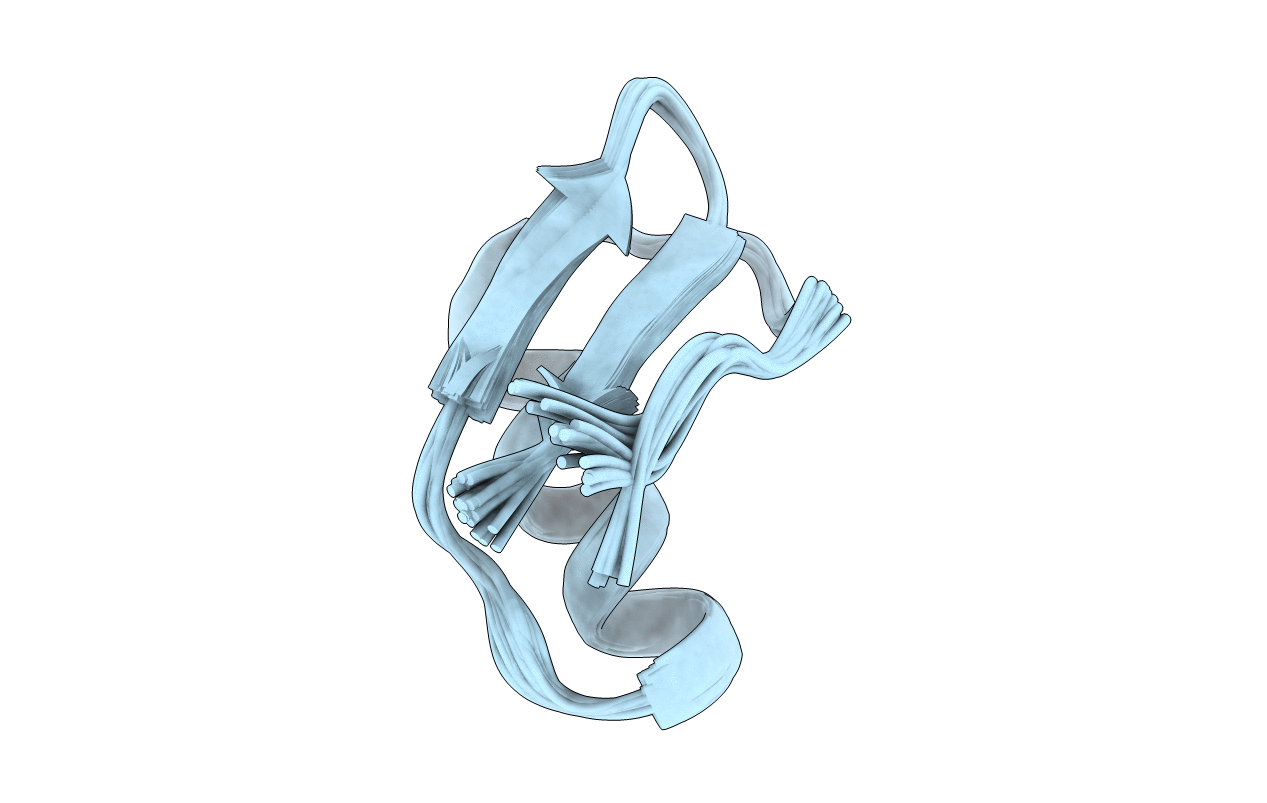
Deposition Date
2002-06-25
Release Date
2004-04-06
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1M2S
Keywords:
Title:
Solution Structure of A New Potassium Channels Blocker from the Venom of Chinese Scorpion Buthus martensi Karsch
Biological Source:
Source Organism(s):
Mesobuthus martensii (Taxon ID: 34649)
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
25
Selection Criteria:
structures with the lowest energy