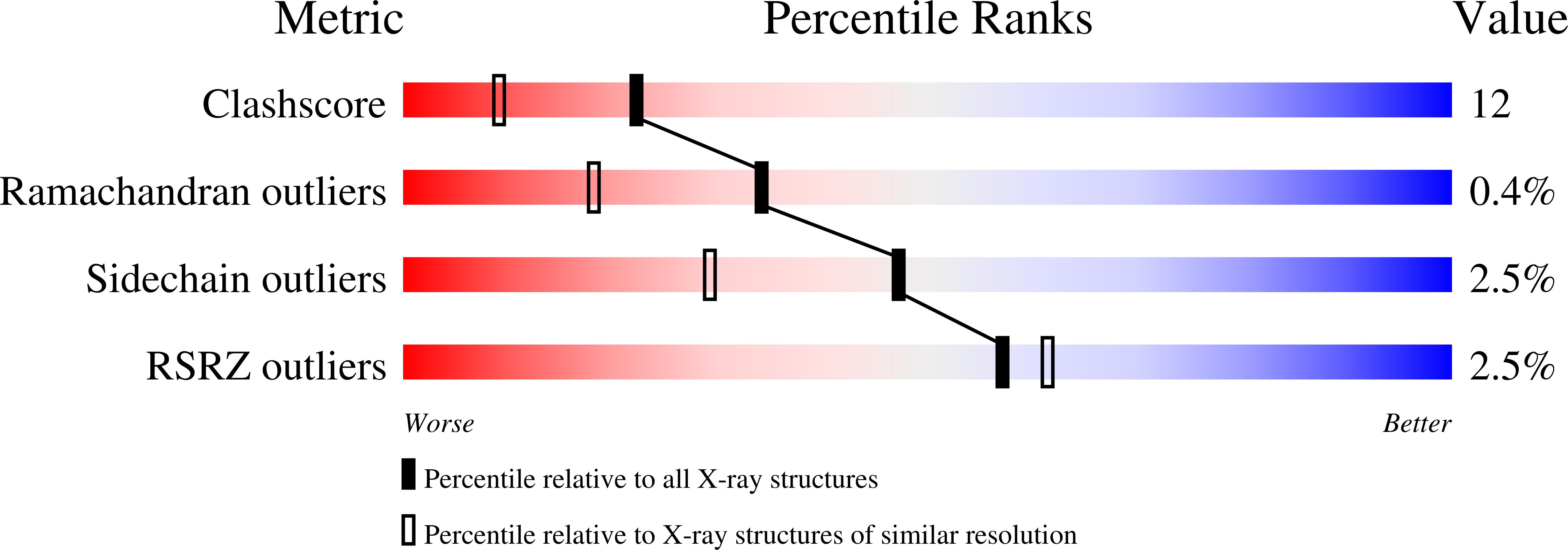Deposition Date
2002-06-17
Release Date
2003-08-05
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1M16
Keywords:
Title:
Human Acidic Fibroblast Growth Factor. 141 Amino Acid Form with Amino Terminal His Tag and Leu 44 Replaced with Phe (L44F), Leu 73 Replaced with Val (L73V), Val 109 Replaced with Leu (V109L) and Cys 117 Replaced with Val (C117V).
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.70 Å
R-Value Free:
0.24
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
C 2 2 21