Deposition Date
1997-09-05
Release Date
1998-01-28
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1LVK
Keywords:
Title:
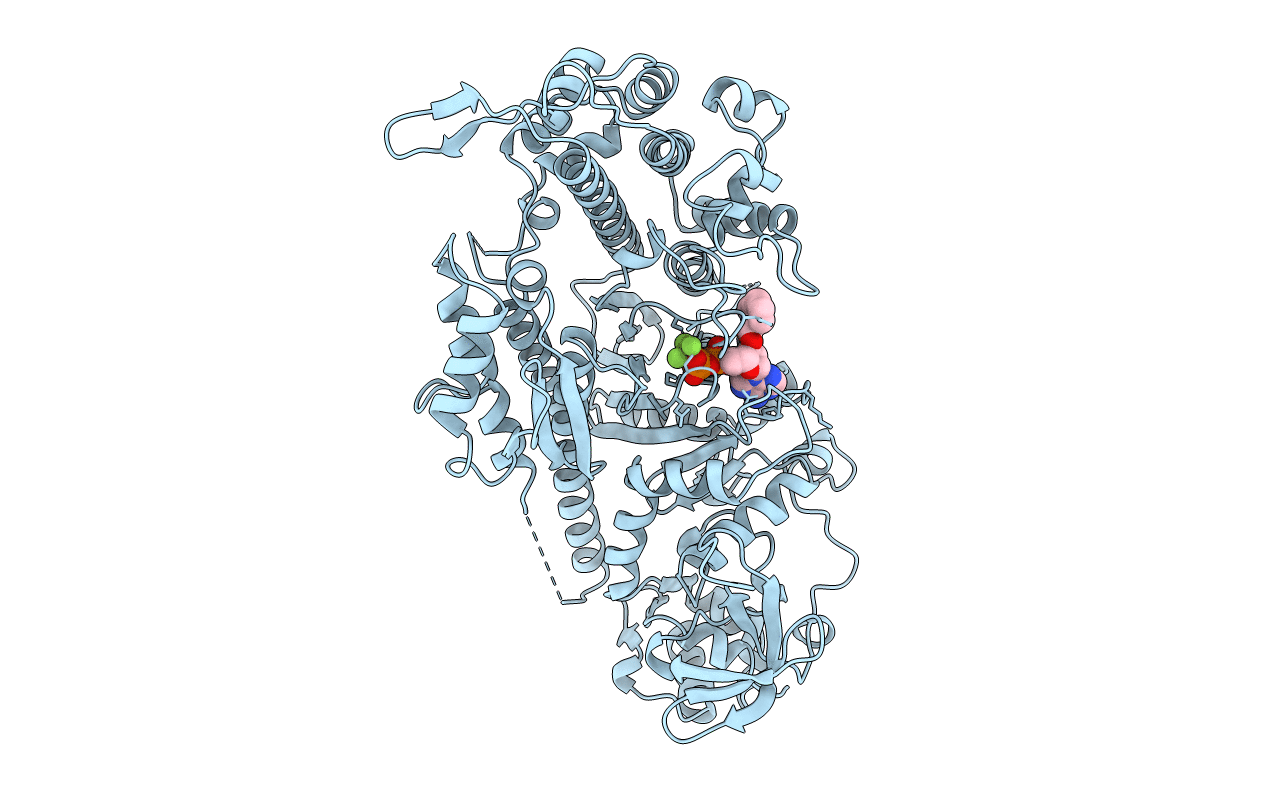
X-RAY CRYSTAL STRUCTURE OF THE MG (DOT) 2'(3')-O-(N-METHYLANTHRANILOYL) NUCLEOTIDE BOUND TO DICTYOSTELIUM DISCOIDEUM MYOSIN MOTOR DOMAIN
Biological Source:
Source Organism:
Dictyostelium discoideum (Taxon ID: 44689)
Host Organism:
Method Details: