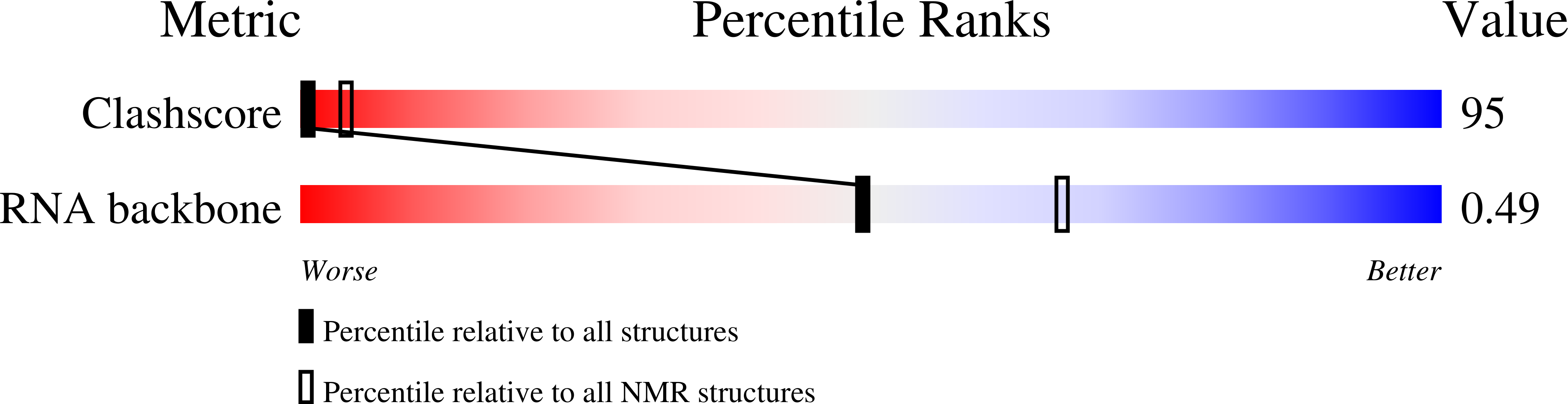
Deposition Date
2002-05-28
Release Date
2002-12-11
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1LVJ
Keywords:
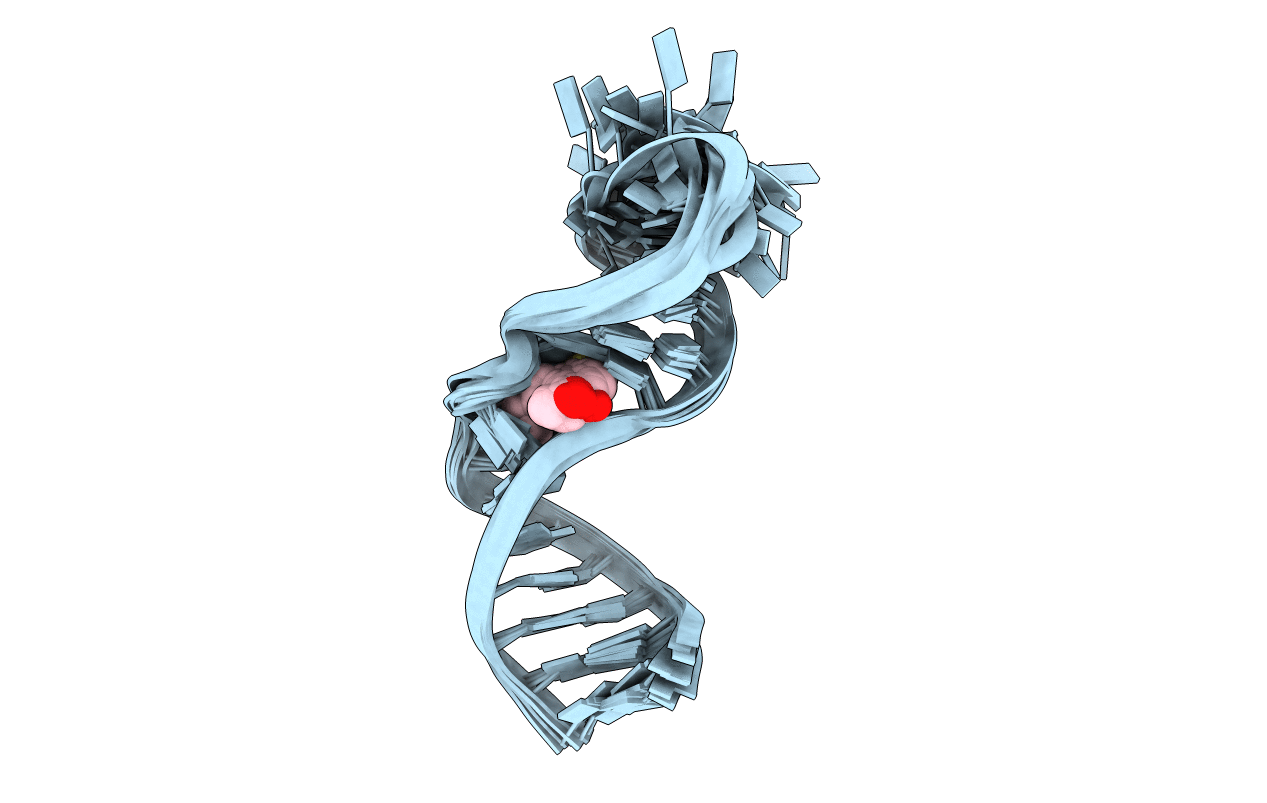
Title:
STRUCTURE OF TAR RNA COMPLEXED WITH A TAT-TAR INTERACTION NANOMOLAR INHIBITOR THAT WAS IDENTIFIED BY COMPUTATIONAL SCREENING
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Method Details:
Experimental Method:
Conformers Calculated:
12
Conformers Submitted:
12
Selection Criteria:
least violations and lowest energy