Deposition Date
2002-05-03
Release Date
2003-06-03
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1LNT
Keywords:
Title:
Crystal Structure of the Highly Conserved RNA Internal Loop of SRP
Method Details:
Experimental Method:
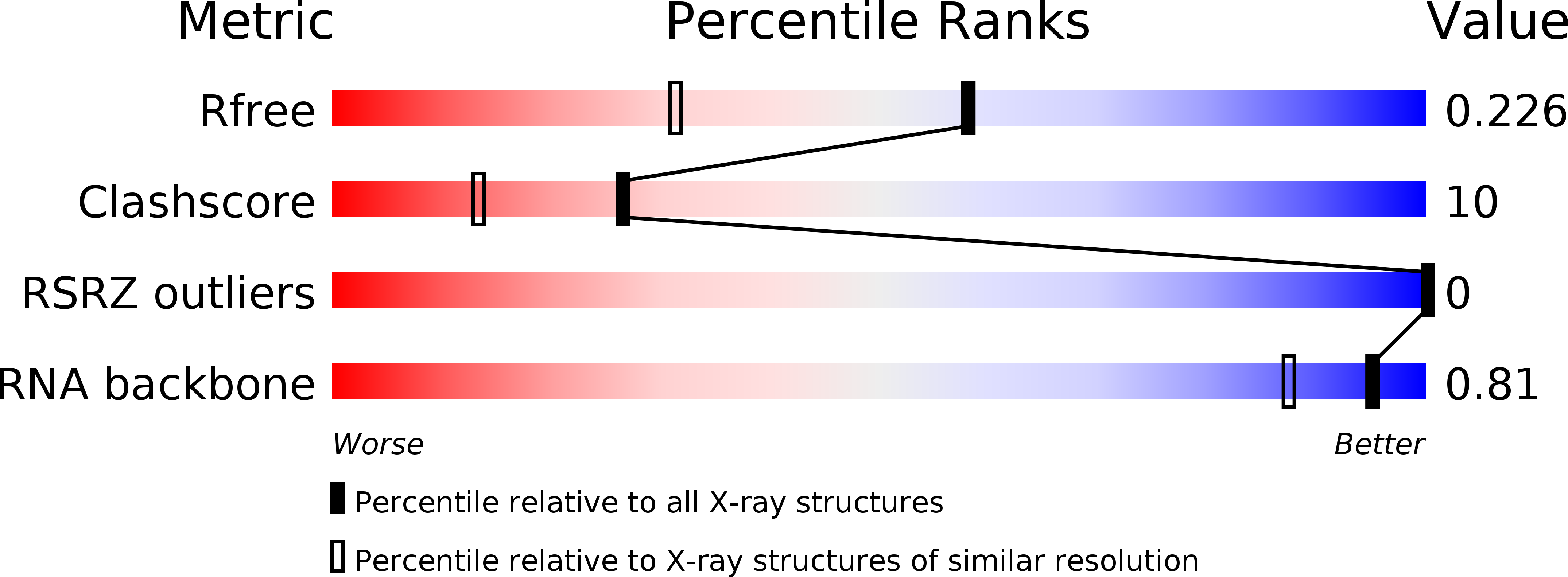
Resolution:
1.70 Å
R-Value Free:
0.22
R-Value Work:
0.2
Space Group:
P 43 2 2