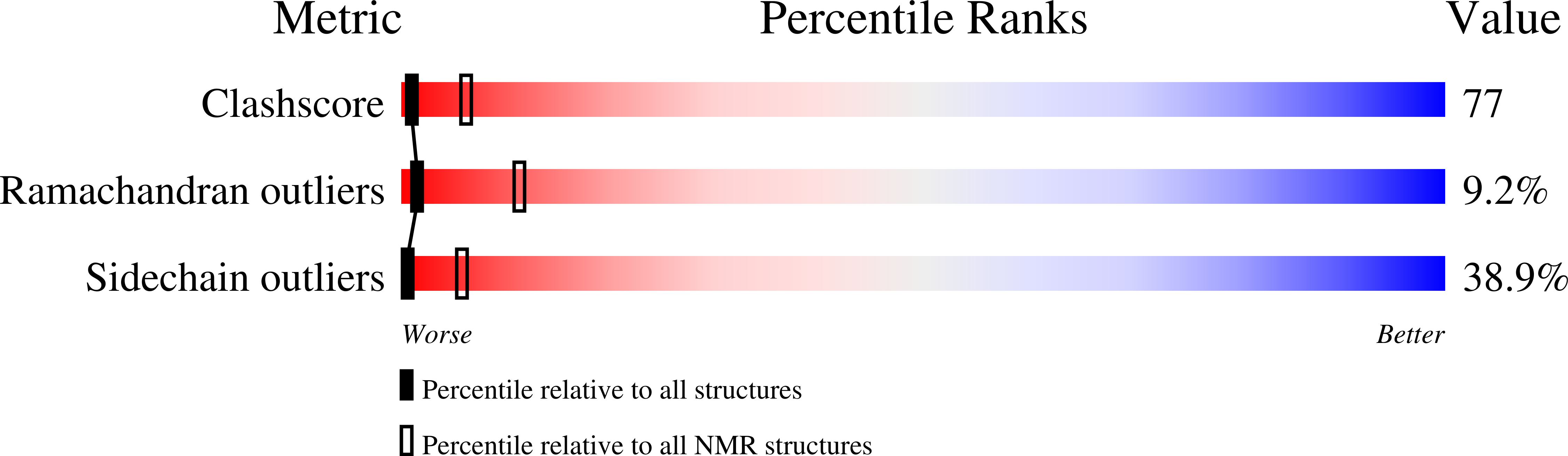
Deposition Date
1998-06-24
Release Date
1998-11-04
Last Version Date
2024-10-23
Entry Detail
PDB ID:
1LFC
Keywords:
Title:
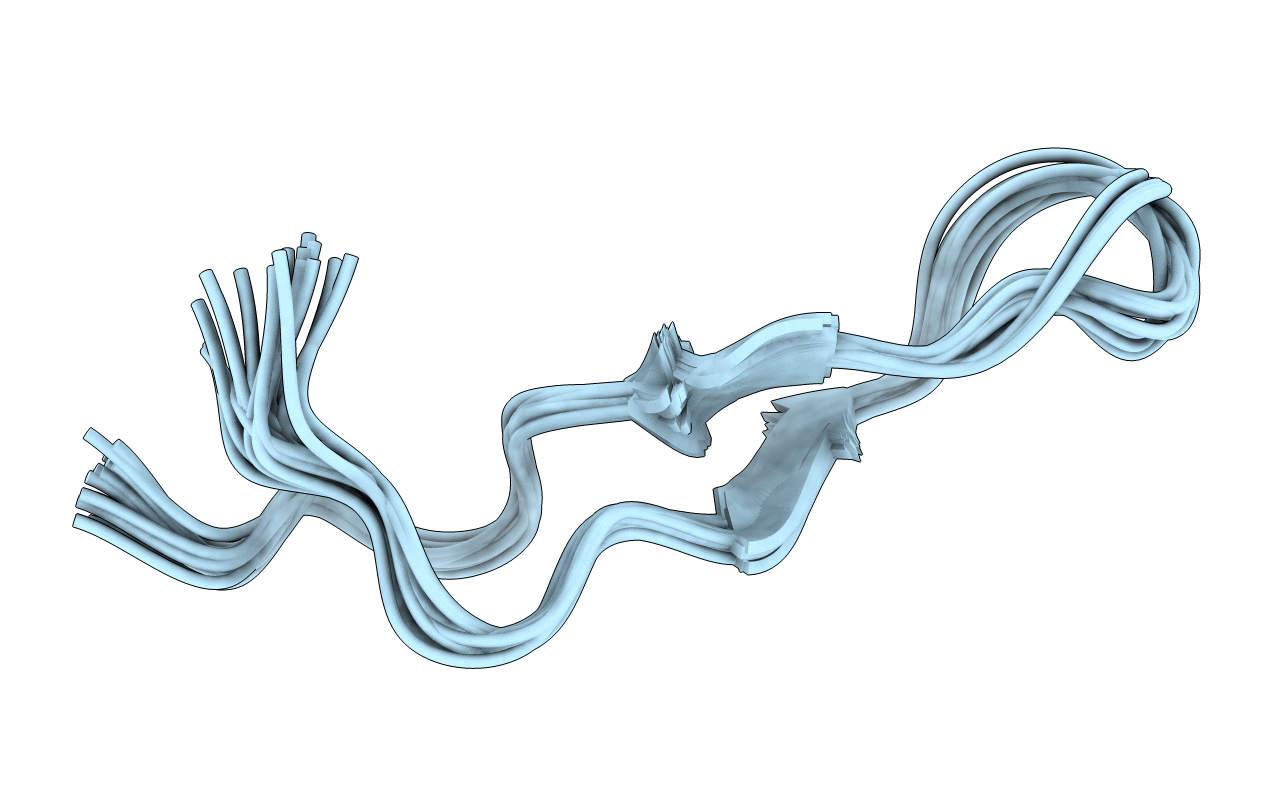
BOVINE LACTOFERRICIN (LFCINB), NMR, 20 STRUCTURES
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
LEAST RESTRAINT VIOLATION