Deposition Date
1993-11-17
Release Date
1994-01-31
Last Version Date
2024-02-14
Entry Detail
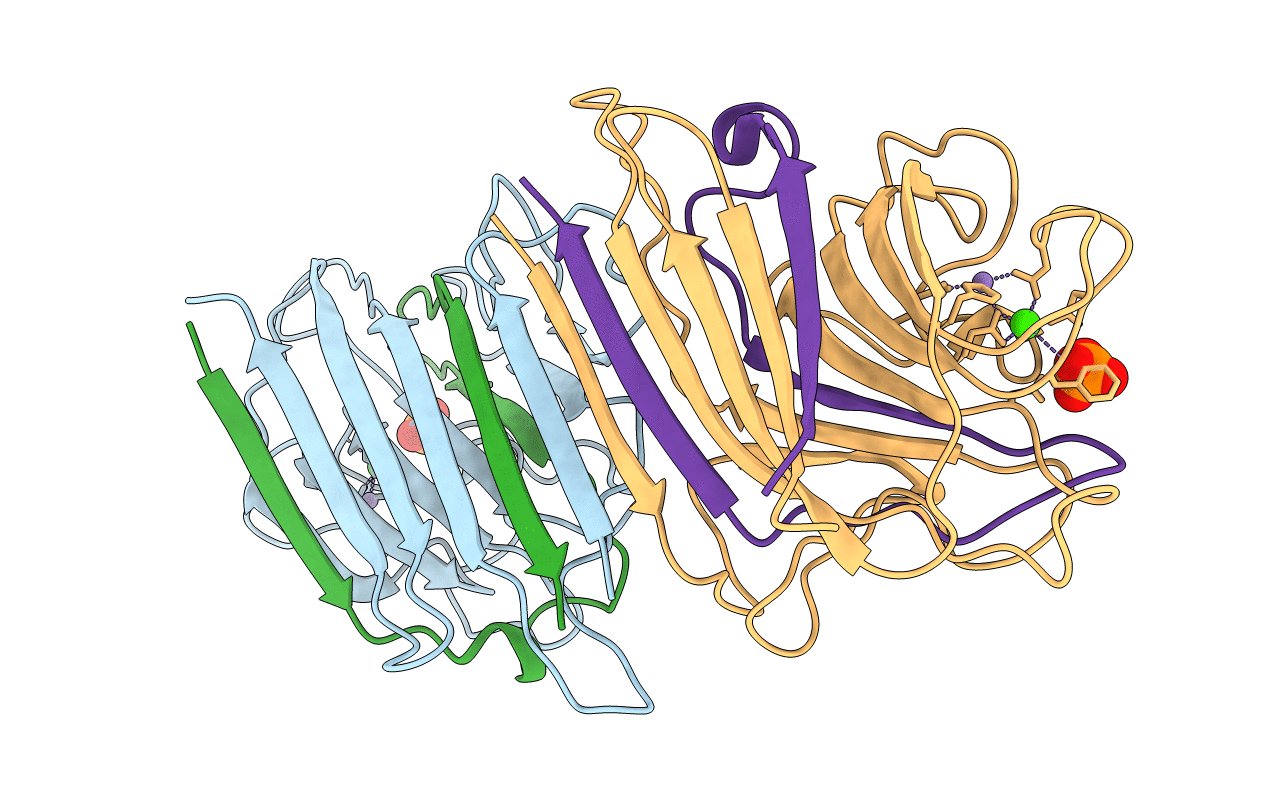
PDB ID:
1LEN
Keywords:
Title:
REFINEMENT OF TWO CRYSTAL FORMS OF LENTIL LECTIN AT 1.8 ANGSTROMS RESOLUTION
Biological Source:
Source Organism(s):
Lens culinaris (Taxon ID: 3864)
Method Details:
Experimental Method:
Resolution:
1.80 Å
R-Value Observed:
0.17
Space Group:
P 1 21 1


