Deposition Date
2002-03-26
Release Date
2002-06-05
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1L9V
Keywords:
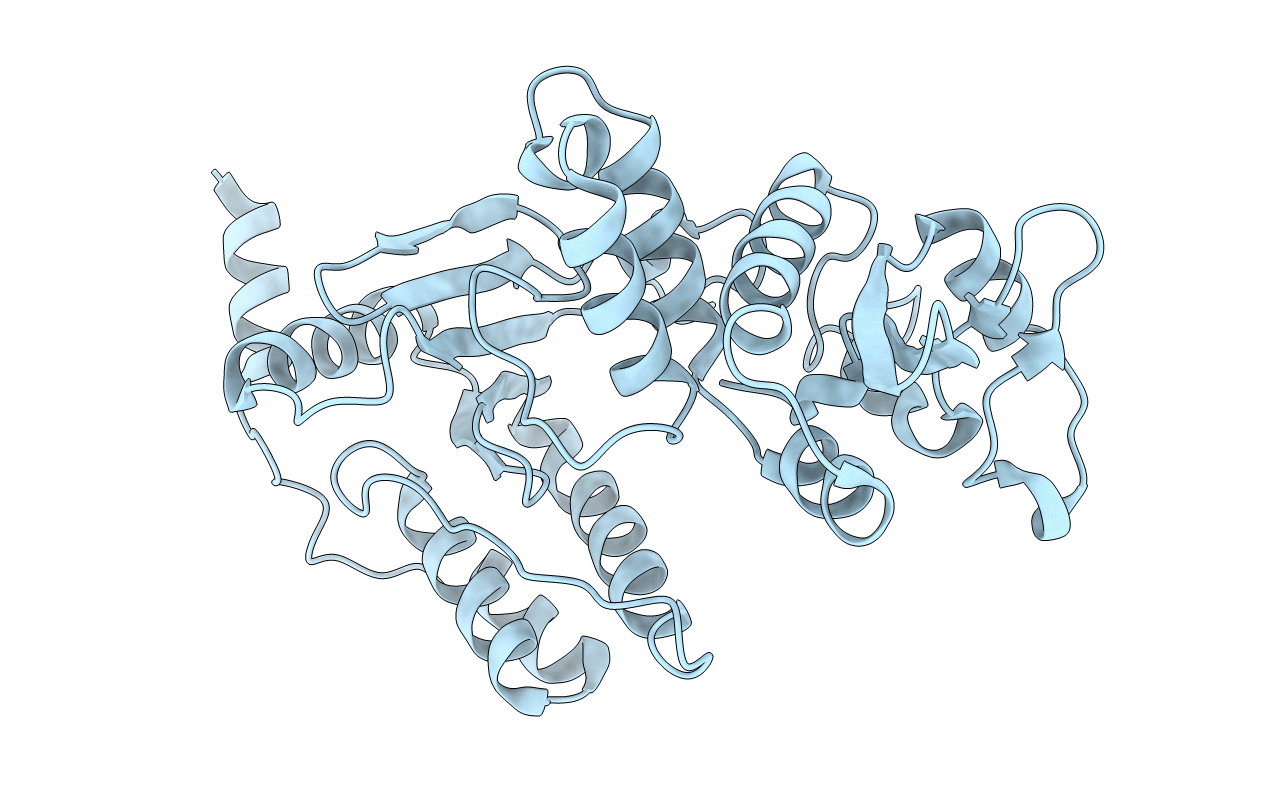
Title:
Non Structural protein encoded by gene segment 8 of rotavirus (NSP2), an NTPase, ssRNA binding and nucleic acid helix-destabilizing protein
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
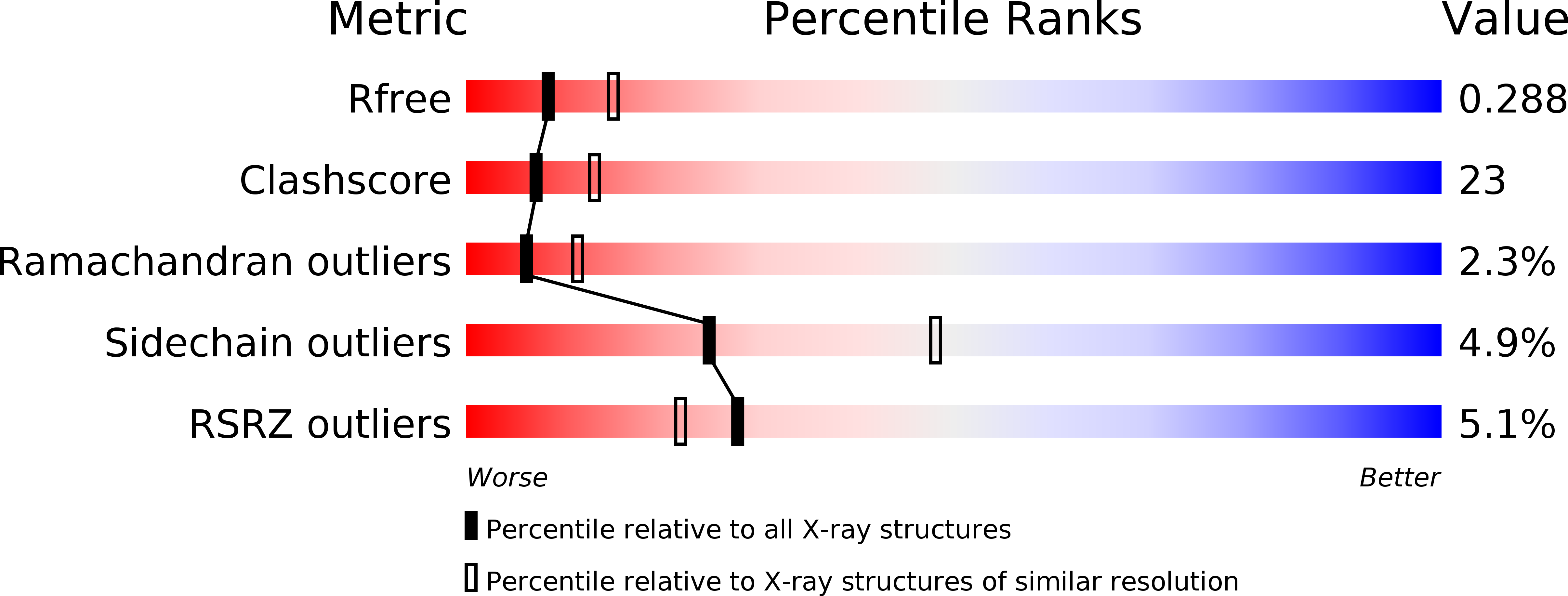
Resolution:
2.60 Å
R-Value Free:
0.28
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
I 4 2 2