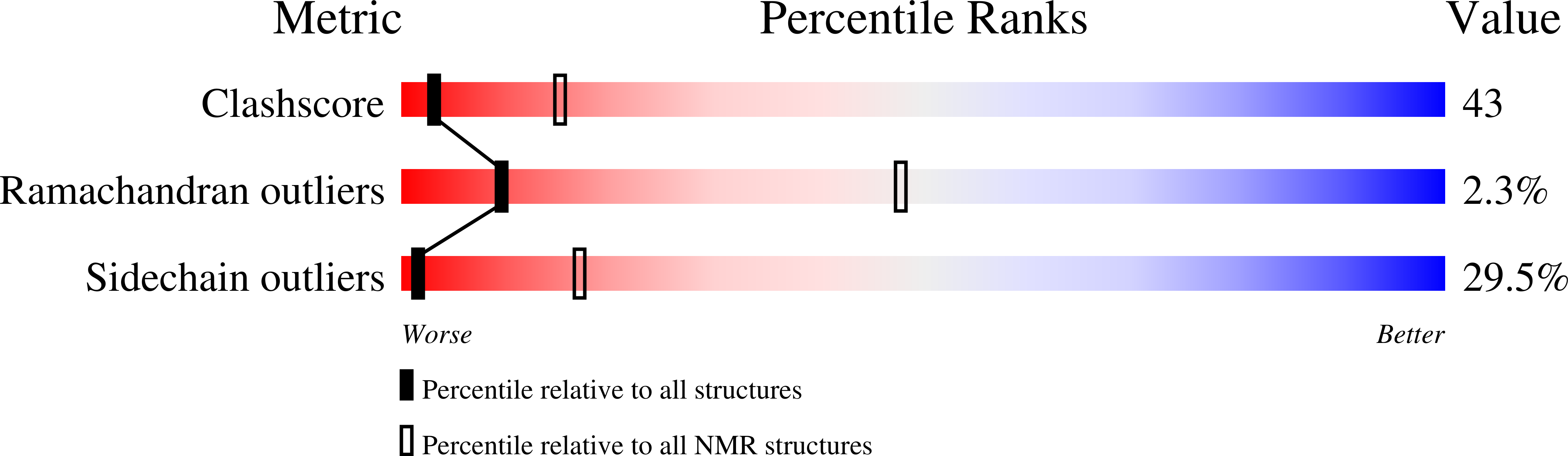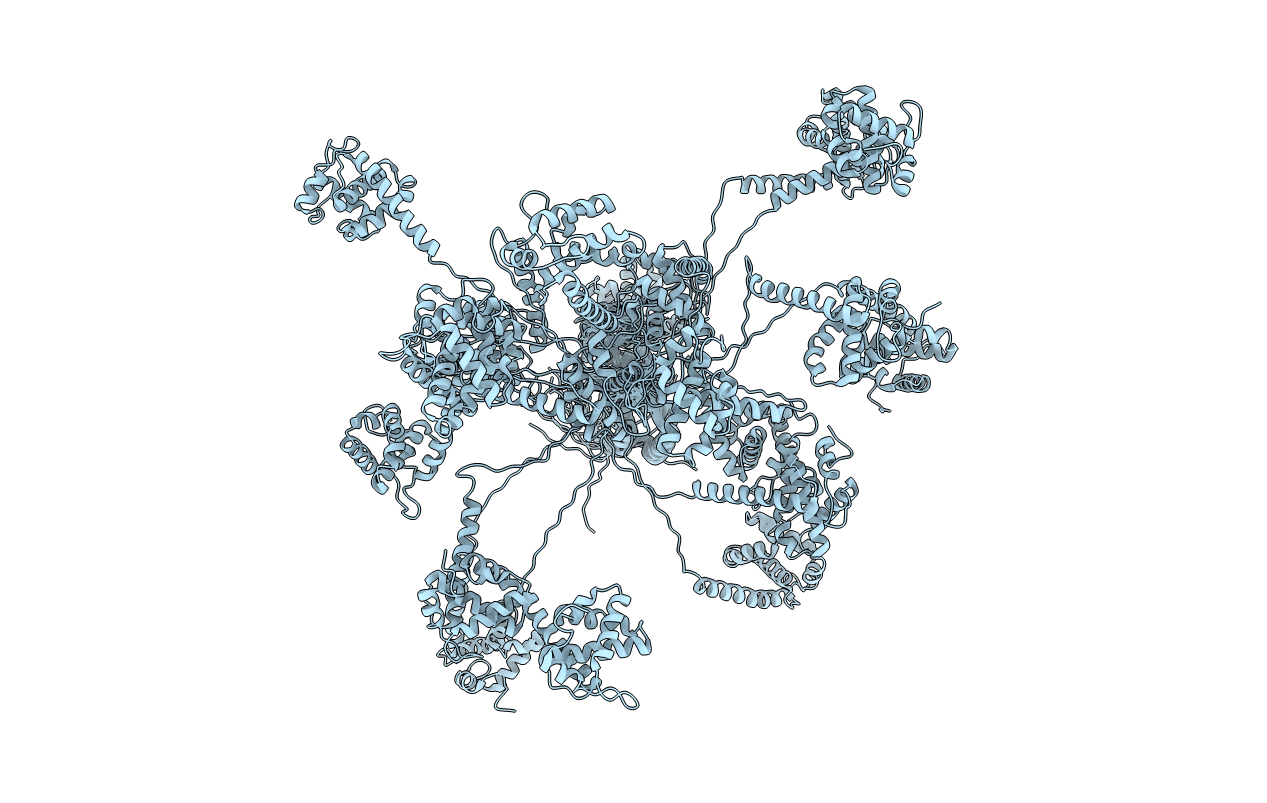
Deposition Date
2002-03-11
Release Date
2002-06-26
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1L6N
Keywords:
Title:
STRUCTURE OF THE N-TERMINAL 283-RESIDUE FRAGMENT OF THE HIV-1 GAG POLYPROTEIN
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
target function