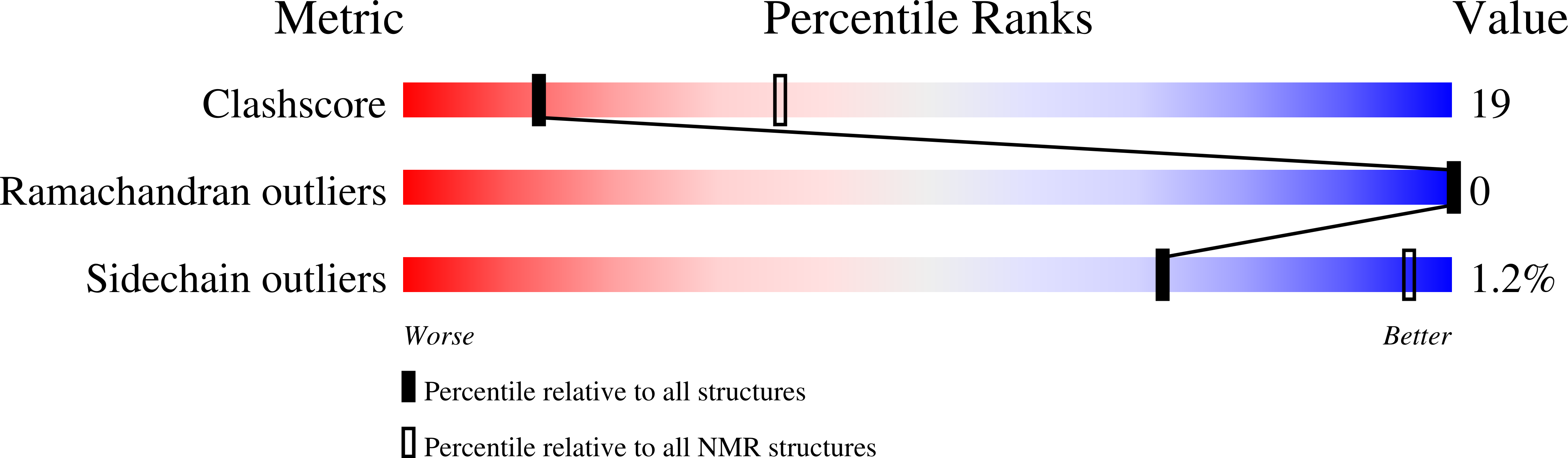
Deposition Date
2002-02-08
Release Date
2002-08-28
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1KZS
Keywords:
Title:
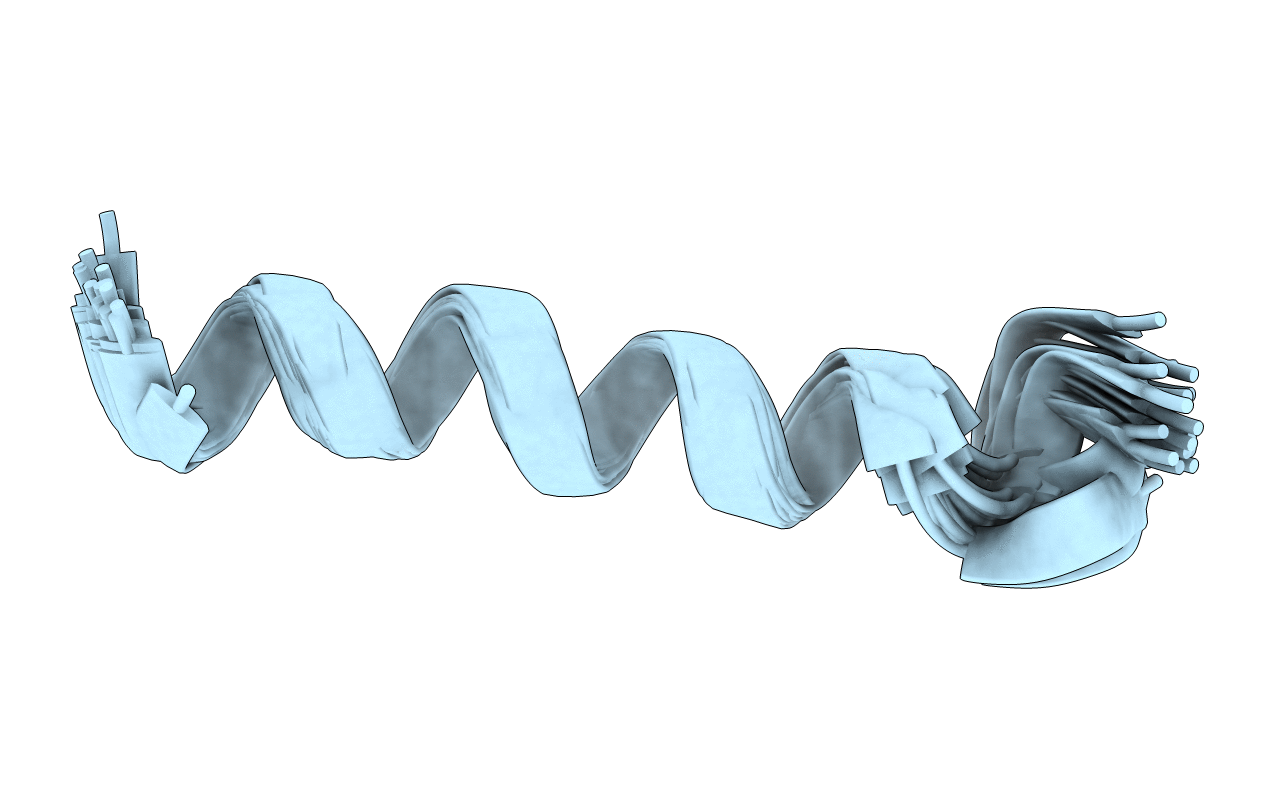
Structure of Human Immunodeficiency Virus Type 1 Vpr(34-51) Peptide in Aqueous TFE Solution
Method Details:
Experimental Method:
Conformers Calculated:
97
Conformers Submitted:
20
Selection Criteria:
structures with the least restraint violations, structures with the lowest energy