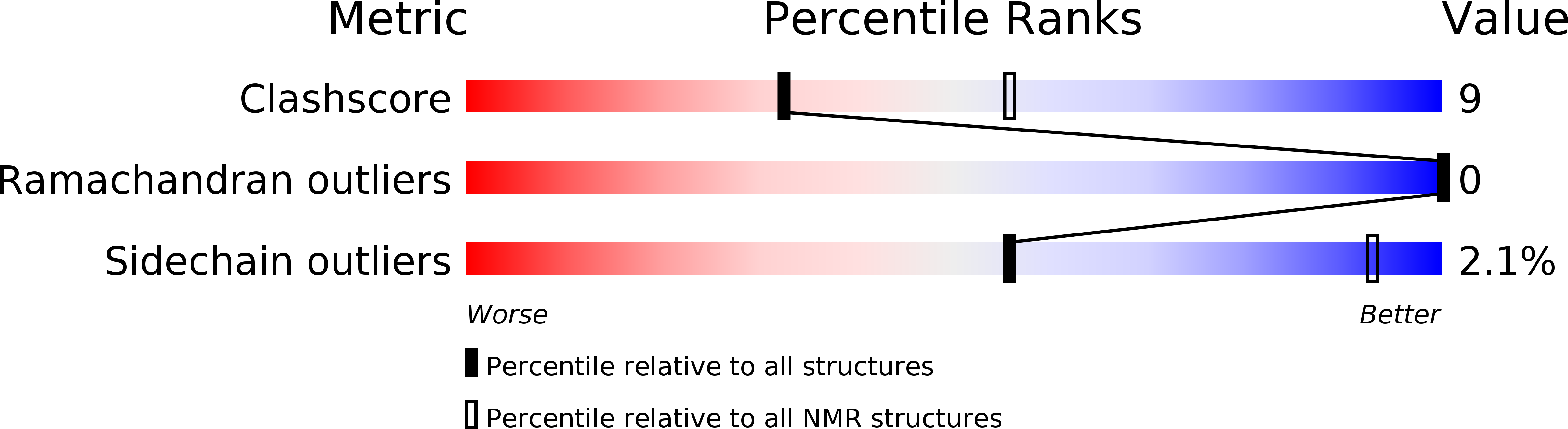Deposition Date
2002-02-04
Release Date
2002-02-20
Last Version Date
2024-10-09
Entry Detail
PDB ID:
1KYJ
Keywords:
Title:
Tumor Associated Mucin Motif from CD43 protein
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
59
Selection Criteria:
No NOE violations greter than 0.15A, no coupling violation greater than 0.5 Hz,
no angle violation greater than 5 deg.