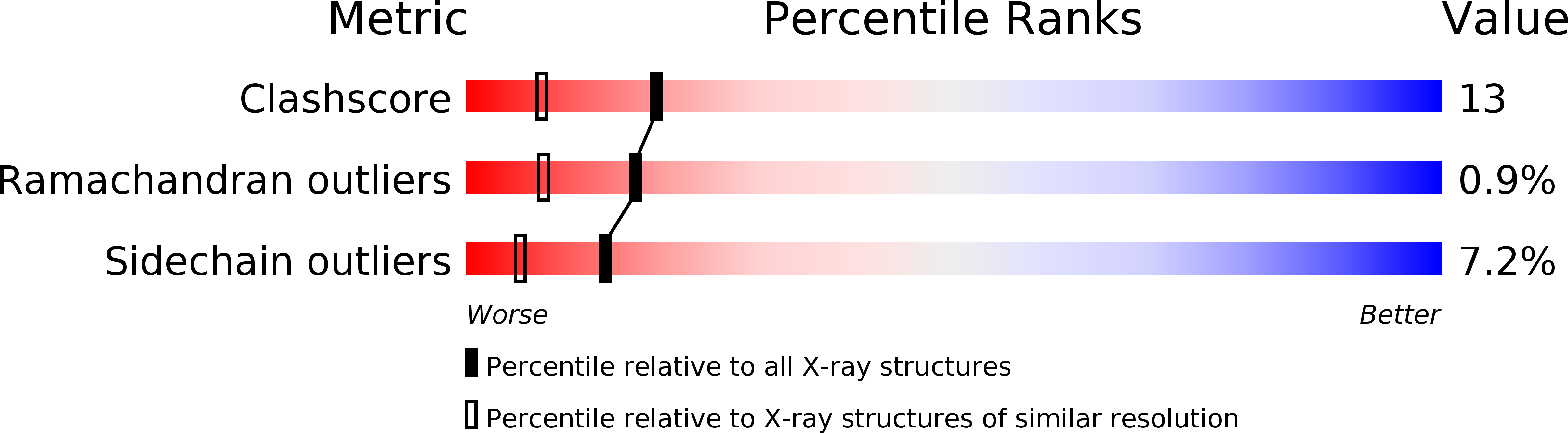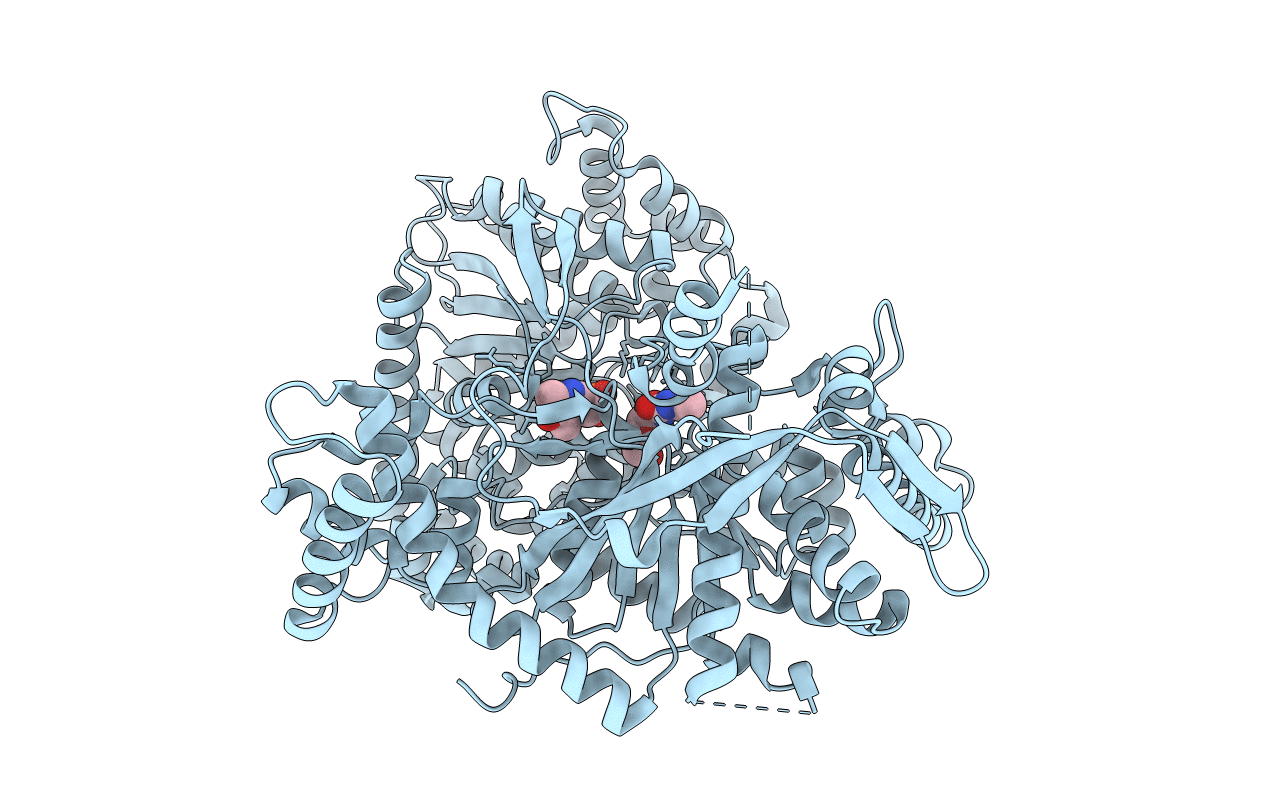
Deposition Date
2002-01-16
Release Date
2002-01-30
Last Version Date
2025-03-26
Entry Detail
PDB ID:
1KTI
Keywords:
Title:
BINDING OF 100 MM N-ACETYL-N'-BETA-D-GLUCOPYRANOSYL UREA TO GLYCOGEN PHOSPHORYLASE B: KINETIC AND CRYSTALLOGRAPHIC STUDIES
Biological Source:
Source Organism(s):
Oryctolagus cuniculus (Taxon ID: 9986)
Method Details:
Experimental Method:
Resolution:
1.97 Å
R-Value Free:
0.21
R-Value Work:
0.19
Space Group:
P 43 21 2