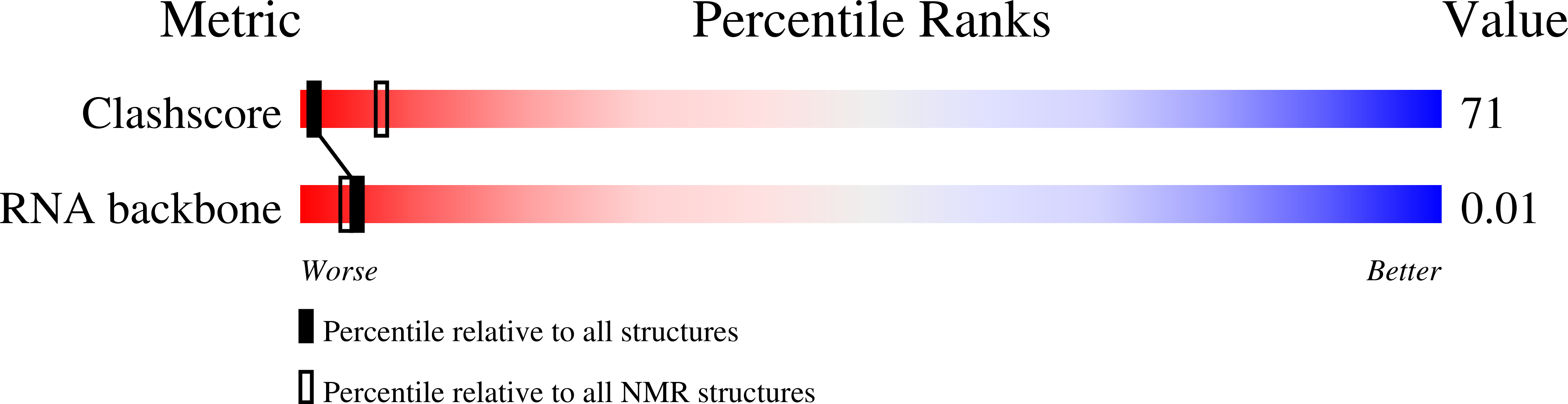
Deposition Date
1999-05-03
Release Date
1999-10-22
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1KOS
Keywords:
Title:
SOLUTION NMR STRUCTURE OF AN ANALOG OF THE YEAST TRNA PHE T STEM LOOP CONTAINING RIBOTHYMIDINE AT ITS NATURALLY OCCURRING POSITION
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
8
Selection Criteria:
LOWEST ENERGY STRUCTURES