Deposition Date
2001-11-16
Release Date
2002-03-06
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1KEQ
Keywords:
Title:
Crystal Structure of F65A/Y131C Carbonic Anhydrase V, covalently modified with 4-chloromethylimidazole
Biological Source:
Source Organism:
Mus musculus (Taxon ID: 10090)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.88 Å
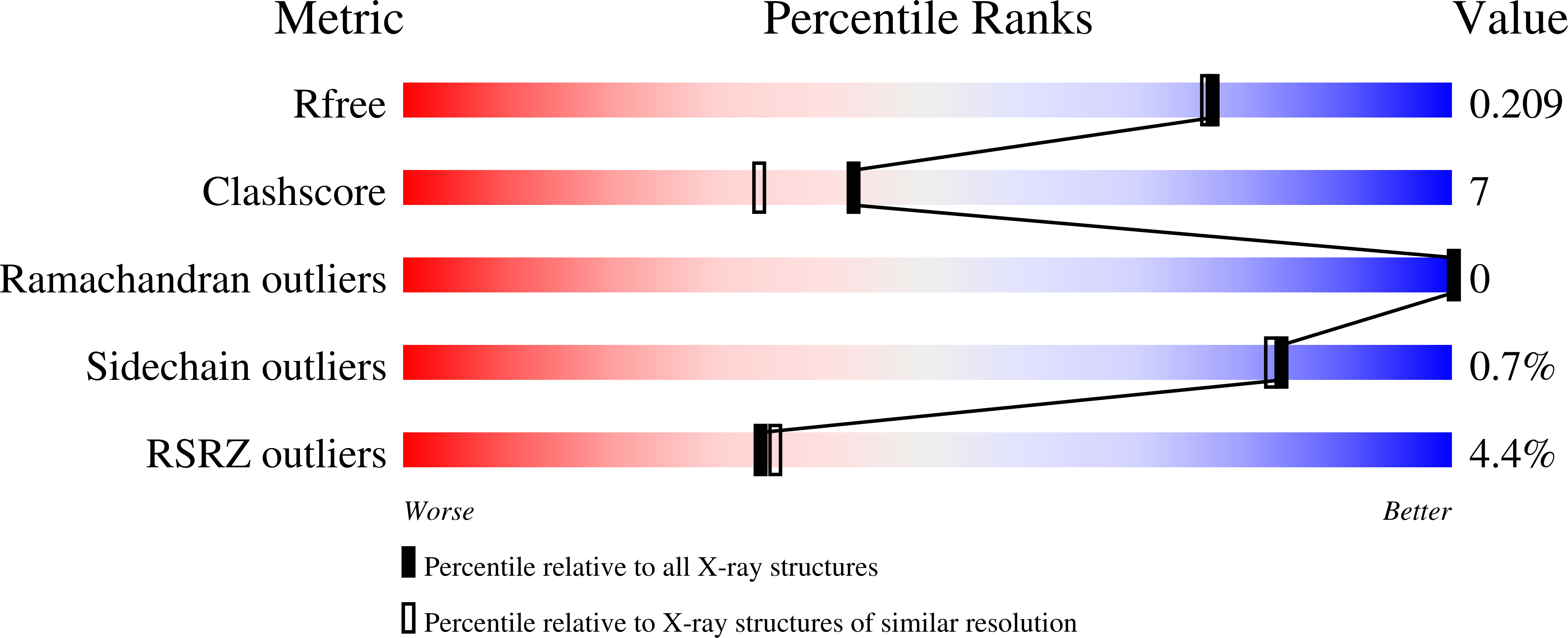
R-Value Free:
0.21
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
C 1 2 1