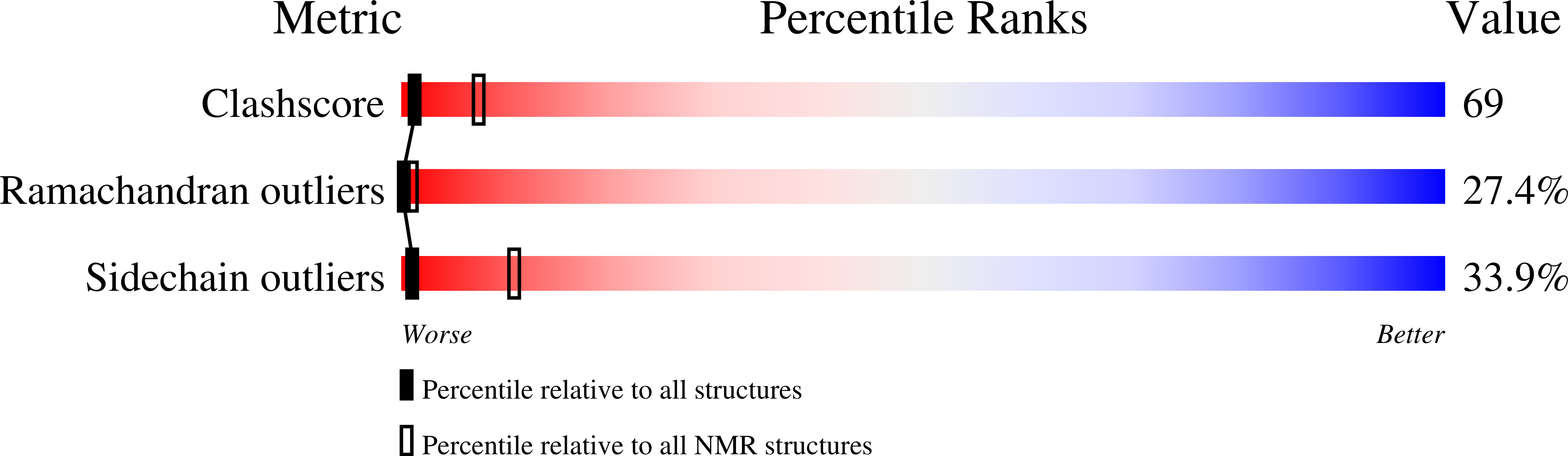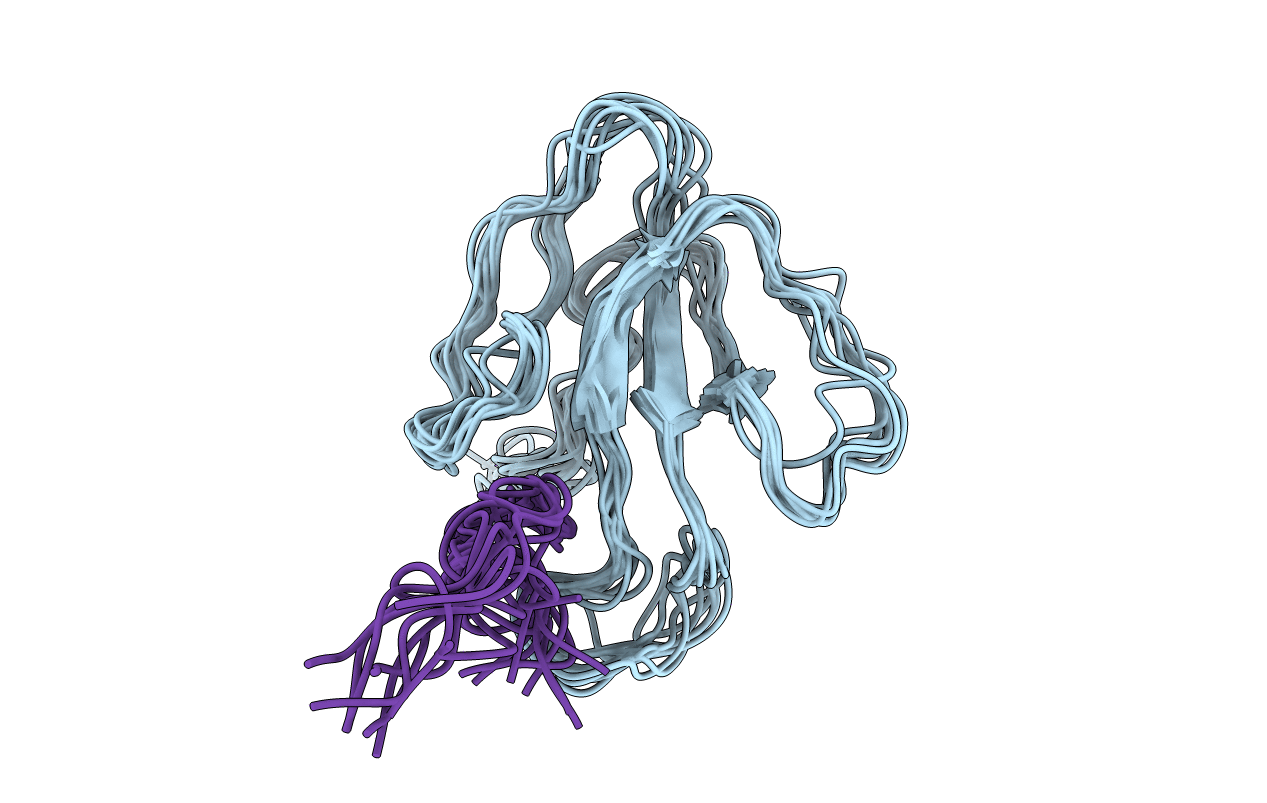
Deposition Date
2001-11-07
Release Date
2002-03-13
Last Version Date
2021-10-27
Entry Detail
PDB ID:
1KC4
Keywords:
Title:
NMR Structural Analysis of the Complex Formed Between alpha-Bungarotoxin and the Principal alpha-Neurotoxin Binding Sequence on the alpha7 Subunit of a Neuronal Nicotinic Acetylcholine Receptor
Biological Source:
Source Organism(s):
Gallus gallus (Taxon ID: 9031)
Bungarus multicinctus (Taxon ID: 8616)
Bungarus multicinctus (Taxon ID: 8616)
Expression System(s):
Method Details:
Experimental Method:
Conformers Submitted:
10
Selection Criteria:
The submitted conformer models are the 10 with the lowest energy from a pool of over 100 accepted structures that had no restraint violations.