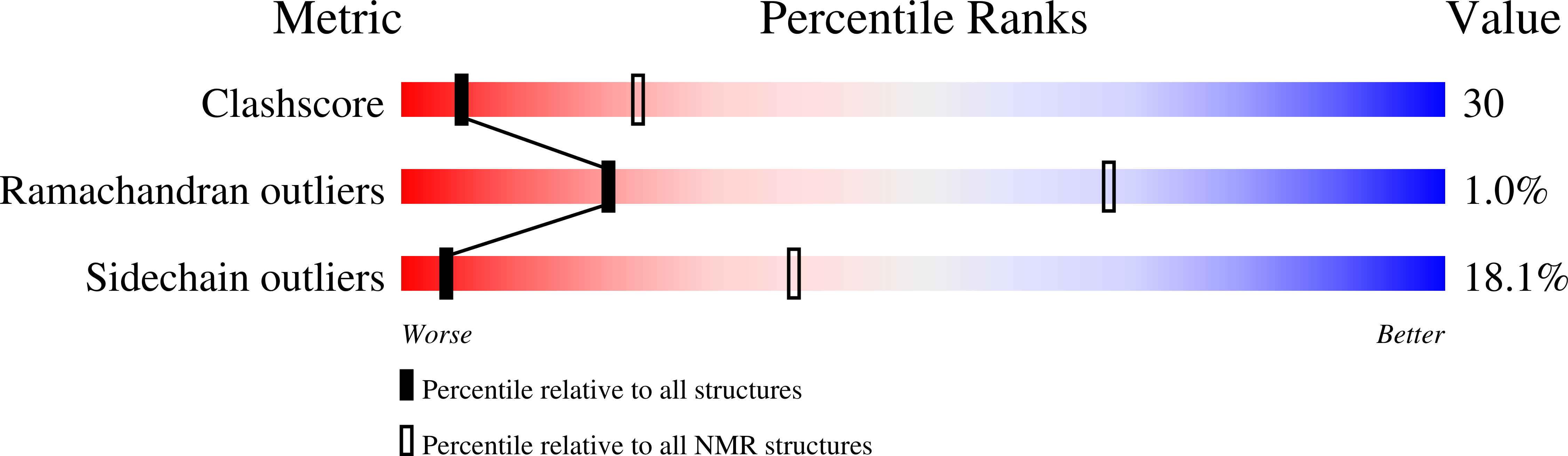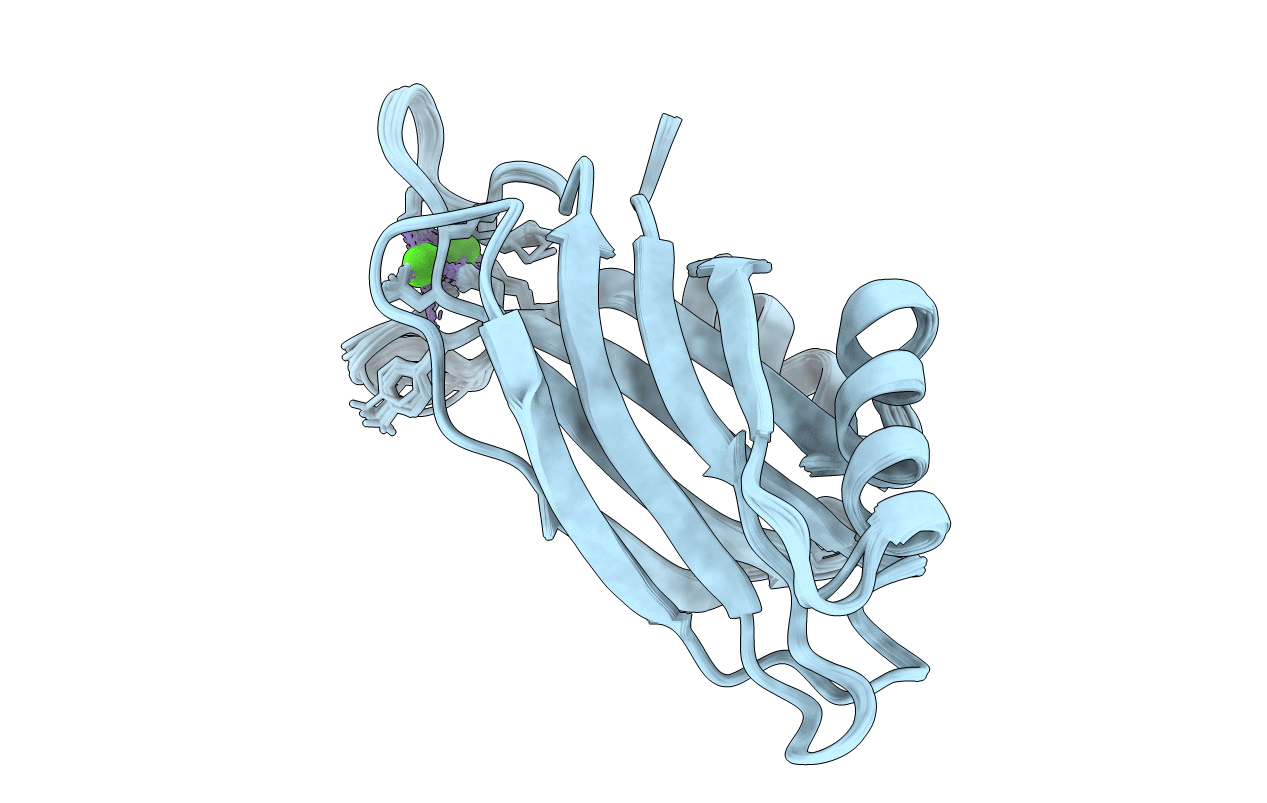
Deposition Date
2001-10-12
Release Date
2002-01-23
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1K5W
Keywords:
Title:
THREE-DIMENSIONAL STRUCTURE OF THE SYNAPTOTAGMIN 1 C2B-DOMAIN: SYNAPTOTAGMIN 1 AS A PHOSPHOLIPID BINDING MACHINE
Biological Source:
Source Organism(s):
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
500
Conformers Submitted:
20
Selection Criteria:
STRUCTURES WITH THE MINIMUM NOE ENERGY