Deposition Date
2001-10-11
Release Date
2002-07-17
Last Version Date
2023-08-16
Entry Detail
PDB ID:
1K5M
Keywords:
Title:
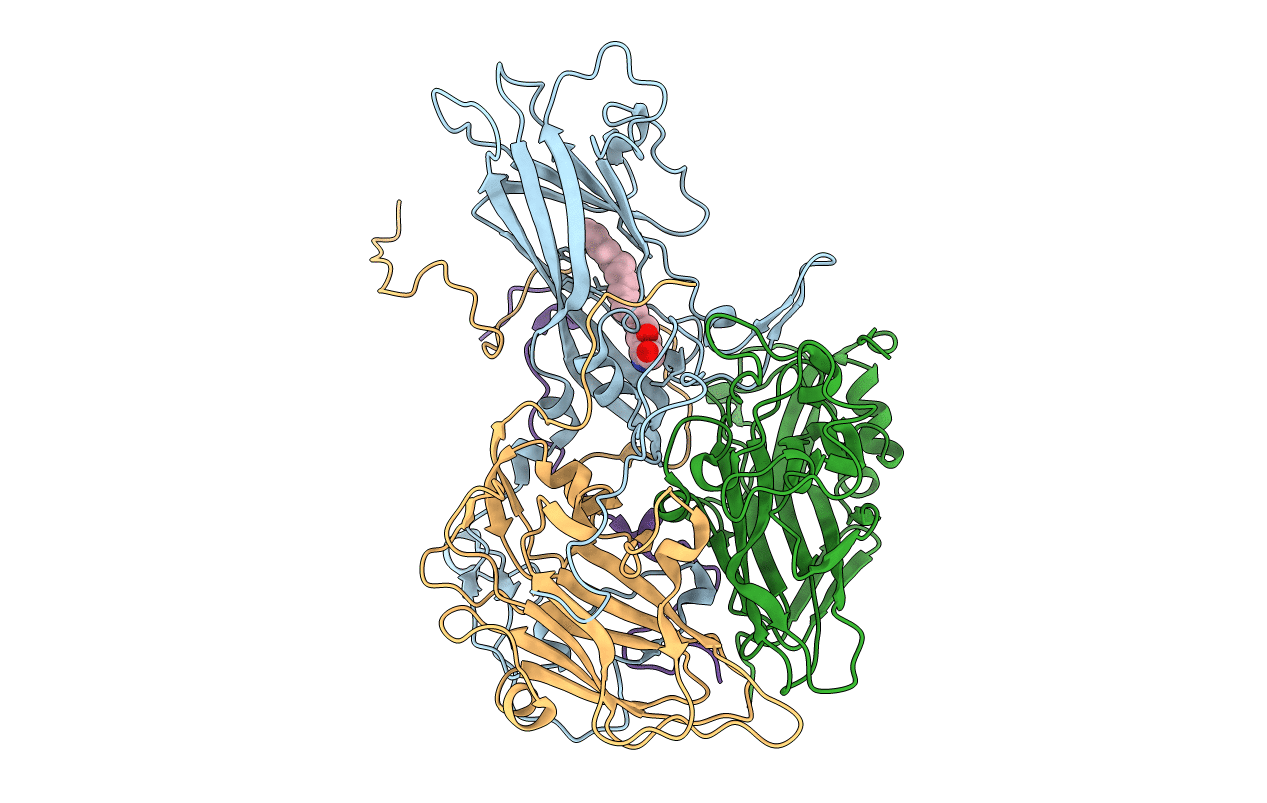
Crystal Structure of a Human Rhinovirus Type 14:Human Immunodeficiency Virus Type 1 V3 Loop Chimeric Virus MN-III-2
Biological Source:
Source Organism(s):
Human rhinovirus 14 (Taxon ID: 12131)
Human immunodeficiency virus type 1 group M subtype B (isolate MN) (Taxon ID: 11696)
Human immunodeficiency virus type 1 group M subtype B (isolate MN) (Taxon ID: 11696)
Expression System(s):
Method Details: