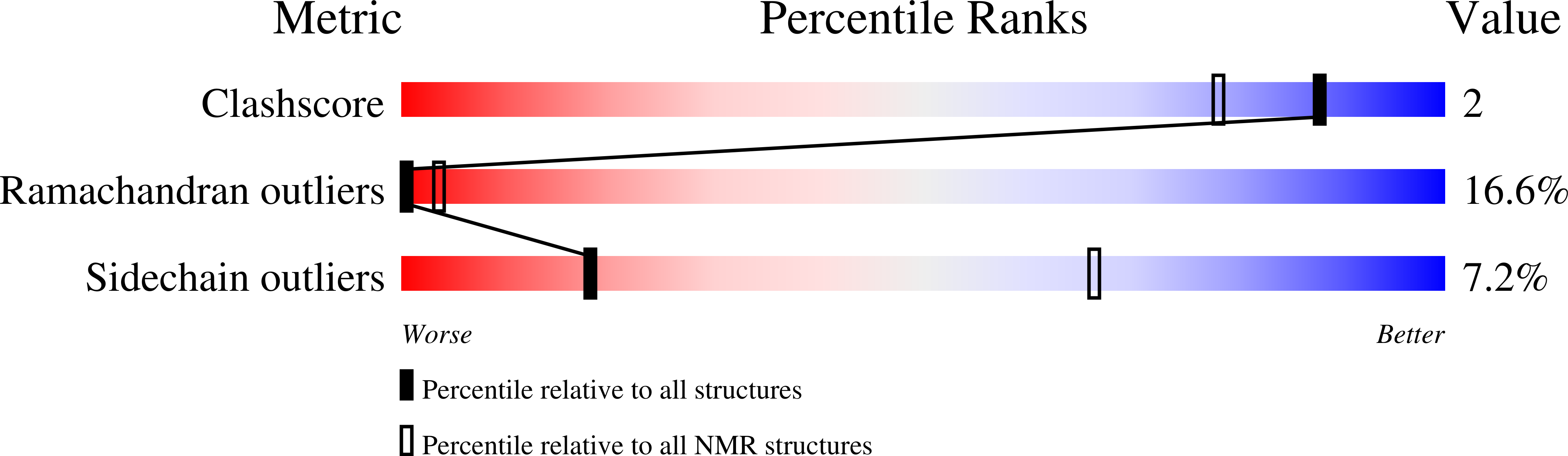
Deposition Date
2001-10-11
Release Date
2002-06-19
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1K5K
Keywords:
Title:
Homonuclear 1H Nuclear Magnetic Resonance Assignment and Structural Characterization of HIV-1 Tat Mal Protein
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
10
Selection Criteria:
back calculated data agree with experimental NOESY spectrum