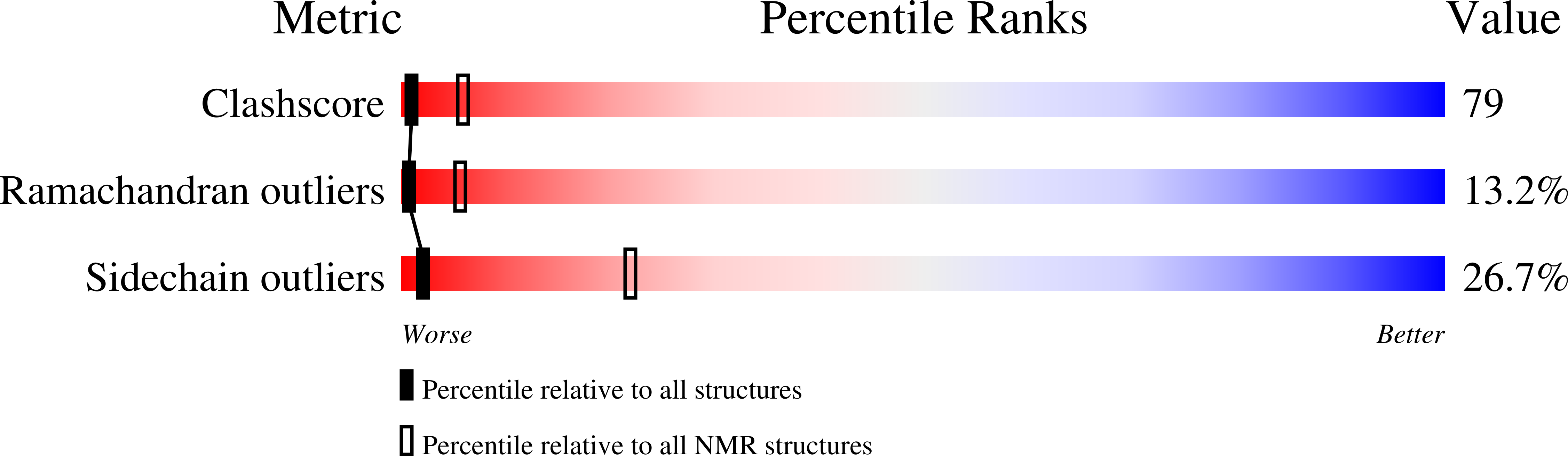Deposition Date
2001-09-18
Release Date
2002-07-10
Last Version Date
2024-02-21
Entry Detail
PDB ID:
1K09
Keywords:
Title:
Solution structure of BetaCore, A Designed Water Soluble Four-Stranded Antiparallel b-sheet Protein
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
20
Selection Criteria:
The submitted conformers are those with no constraint violations greater than 0.5 angstroms for NOEs and 5 degrees for dihedrals. They are also the ones with best covalent geometry.