Deposition Date
2001-08-27
Release Date
2001-09-19
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1JUT
Keywords:
Title:
E. coli Thymidylate Synthase Bound to dUMP and LY338529, A Pyrrolo(2,3-d)pyrimidine-based Antifolate
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
Expression System(s):
Method Details:
Experimental Method:
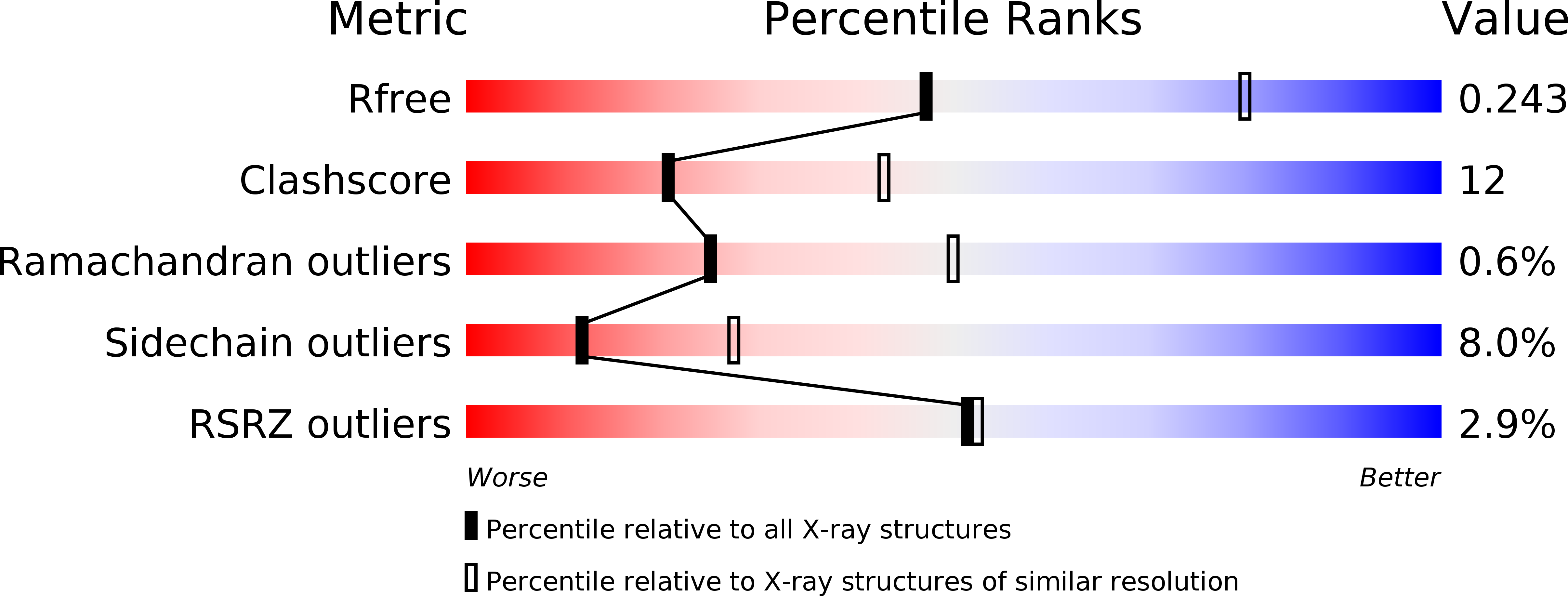
Resolution:
2.70 Å
R-Value Free:
0.25
R-Value Work:
0.21
R-Value Observed:
0.22
Space Group:
P 63