Deposition Date
2001-08-20
Release Date
2001-12-19
Last Version Date
2023-08-16
Entry Detail
PDB ID:
1JTC
Keywords:
Title:
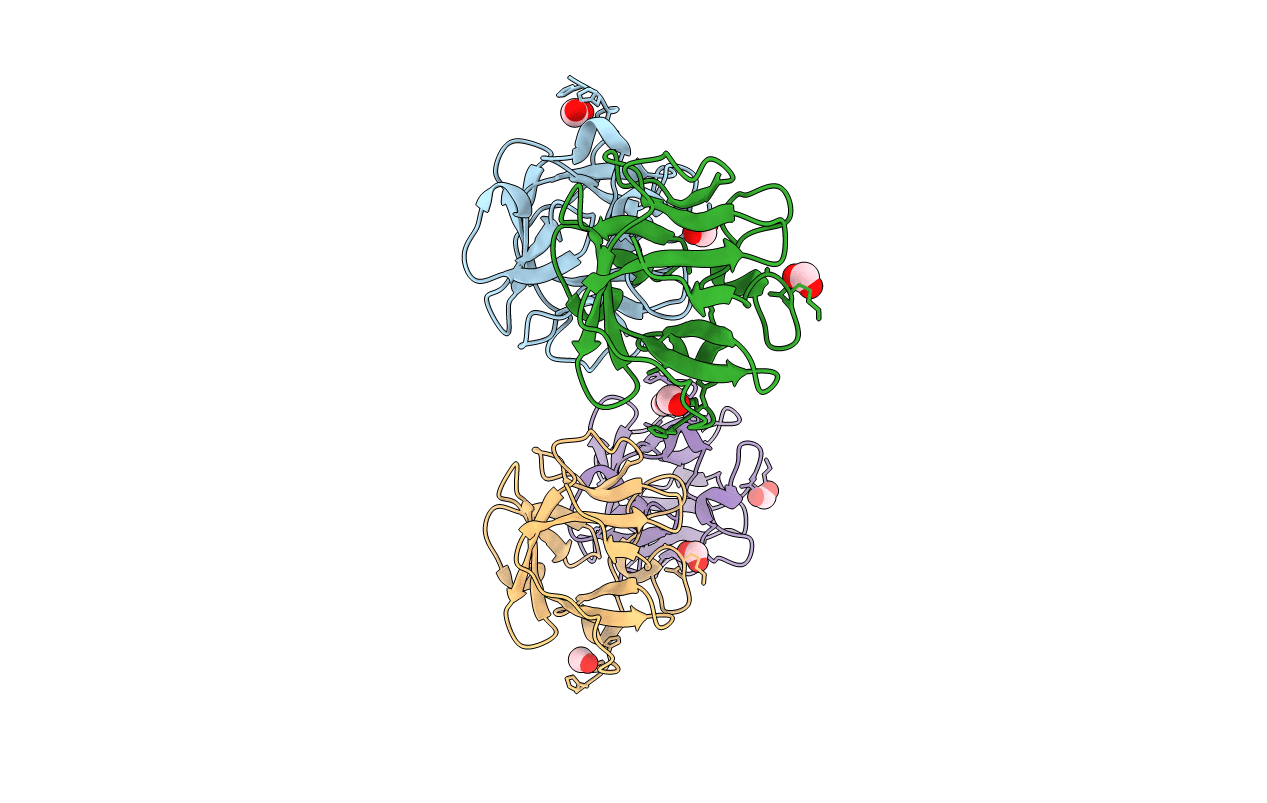
Human Acidic Fibroblast Growth Factor. 141 Amino Acid Form with Amino Terminal His Tag AND LEU 44 REPLACED BY PHE (L44F)
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details: