Deposition Date
2001-07-19
Release Date
2001-10-10
Last Version Date
2023-08-16
Entry Detail
PDB ID:
1JML
Keywords:
Title:
Conversion of Monomeric Protein L to an Obligate Dimer by Computational Protein Design
Biological Source:
Source Organism(s):
Finegoldia magna (Taxon ID: 334413)
Expression System(s):
Method Details:
Experimental Method:
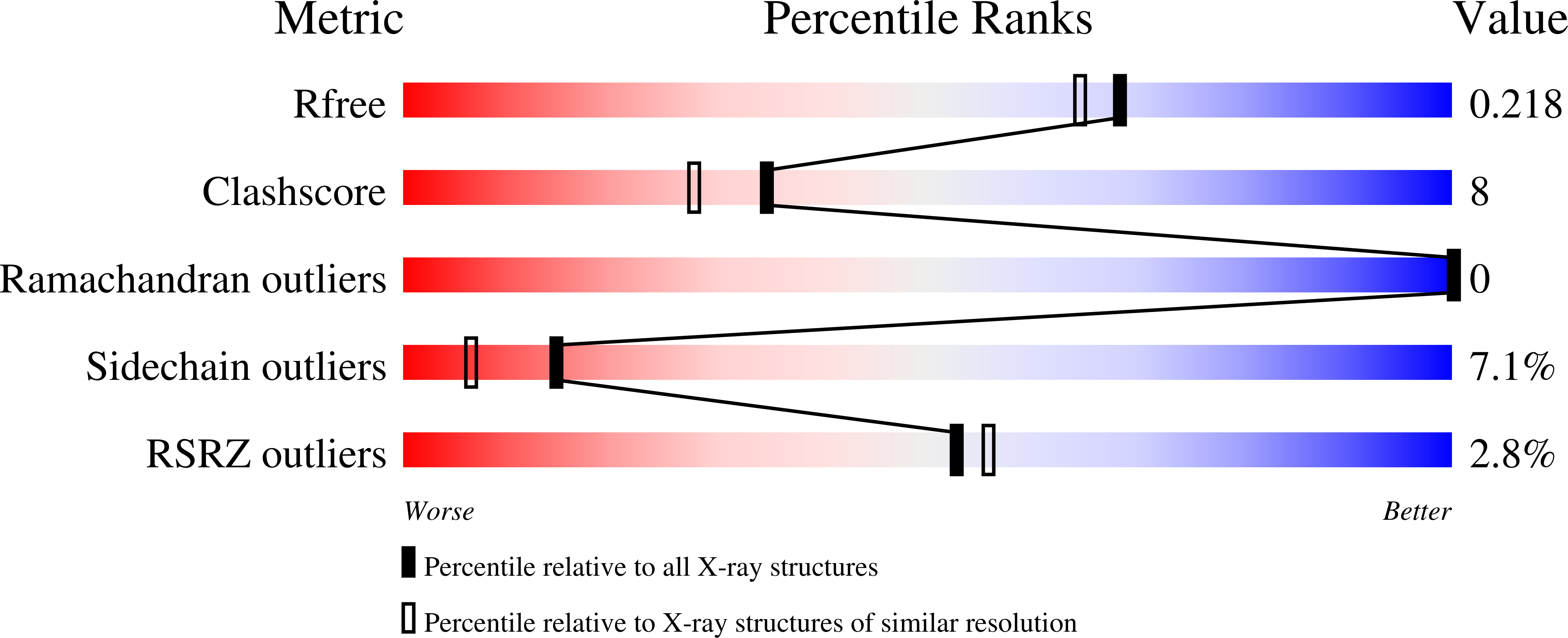
Resolution:
1.90 Å
R-Value Free:
0.21
R-Value Work:
0.19
Space Group:
C 2 2 21