Deposition Date
2001-07-04
Release Date
2001-08-22
Last Version Date
2024-05-01
Entry Detail
PDB ID:
1JJD
Keywords:
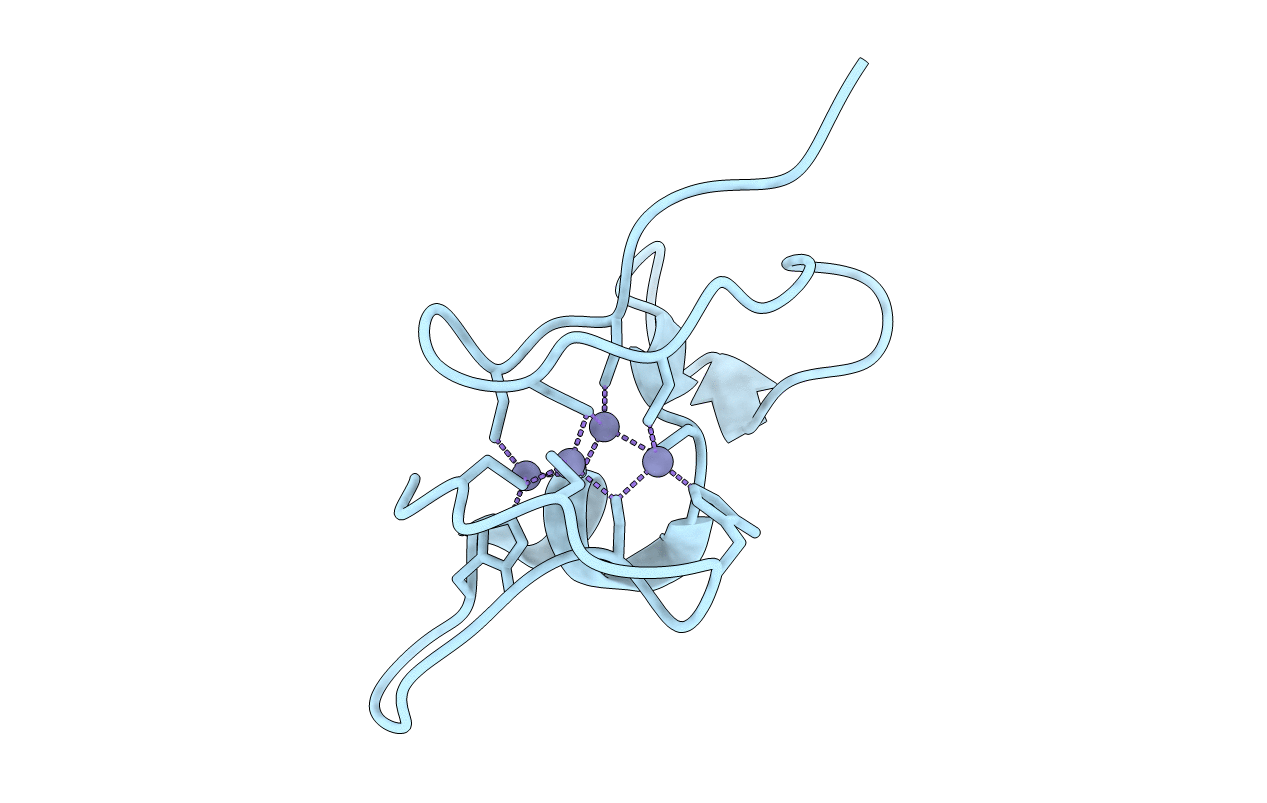
Title:
NMR structure of the Cyanobacterial Metallothionein SmtA
Biological Source:
Source Organism:
Synechococcus elongatus (Taxon ID: 1140)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
500
Conformers Submitted:
1
Selection Criteria:
DIANA target function


