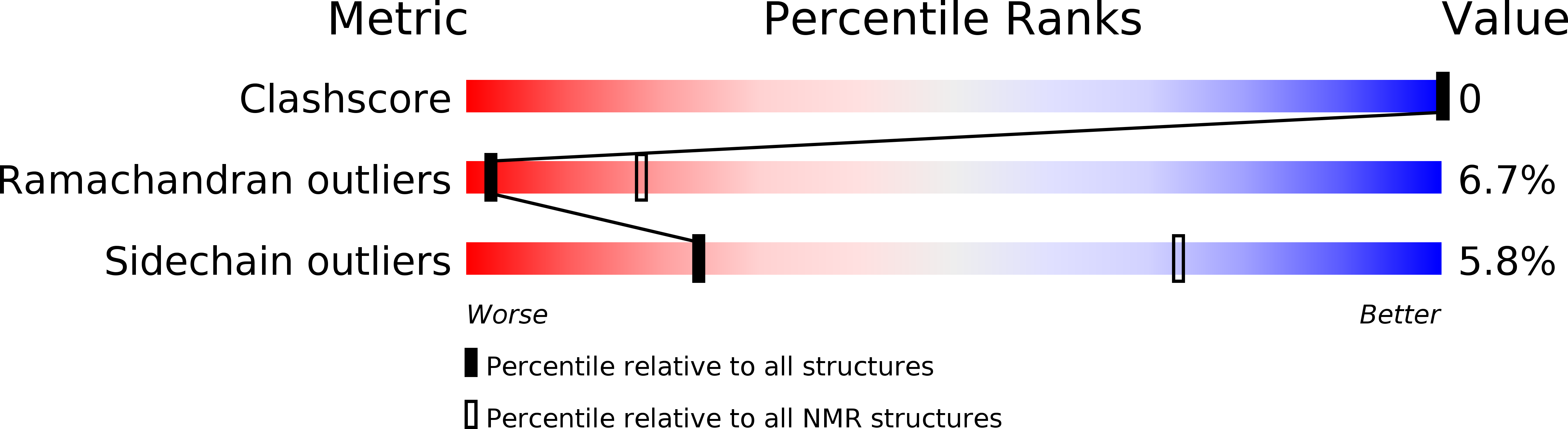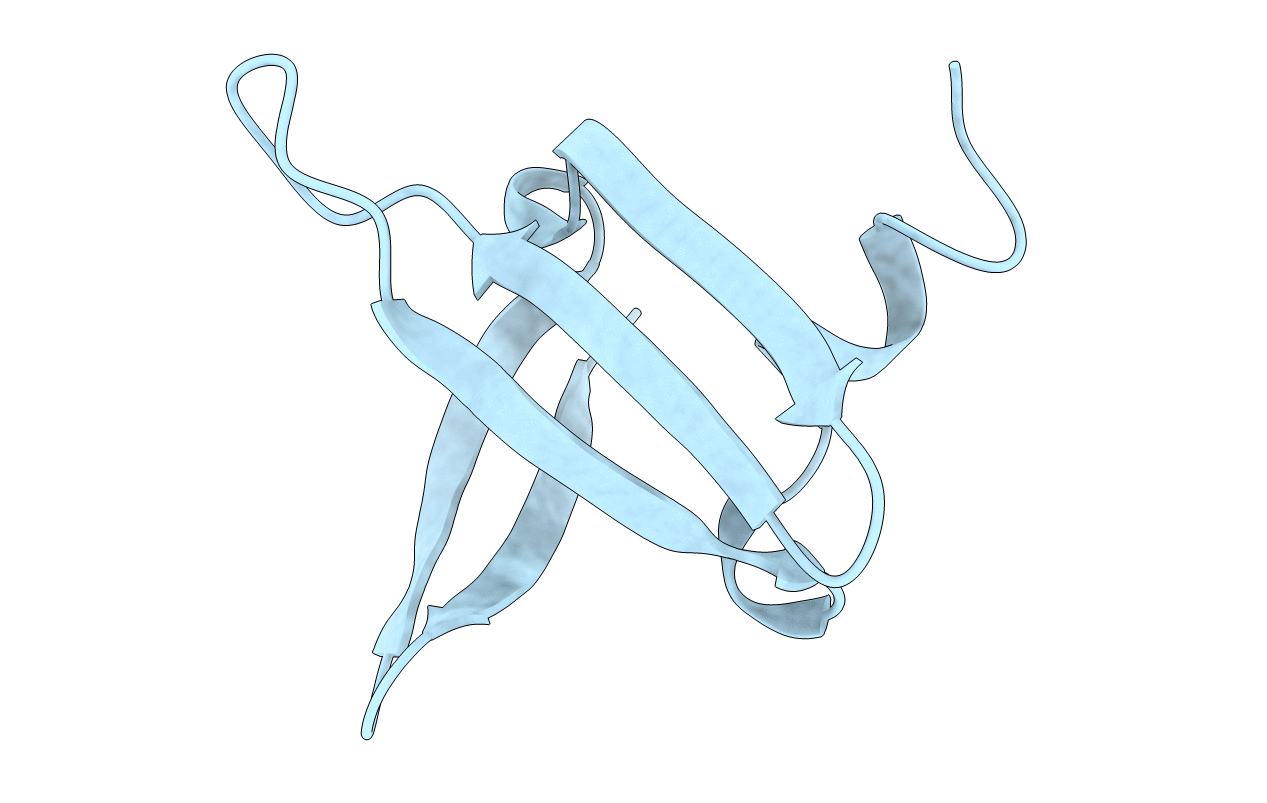
Deposition Date
1998-07-20
Release Date
1998-10-14
Last Version Date
2024-05-01
Entry Detail
PDB ID:
1JIC
Keywords:
Title:
SOLUTION NMR STRUCTURE OF RECOMBINANT SSO7D WITH RNASE ACTIVITY, MINIMIZED AVERAGE STRUCTURE
Biological Source:
Source Organism(s):
Sulfolobus solfataricus (Taxon ID: 2287)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
35
Conformers Submitted:
1
Selection Criteria:
LOWEST ENERGY TERM