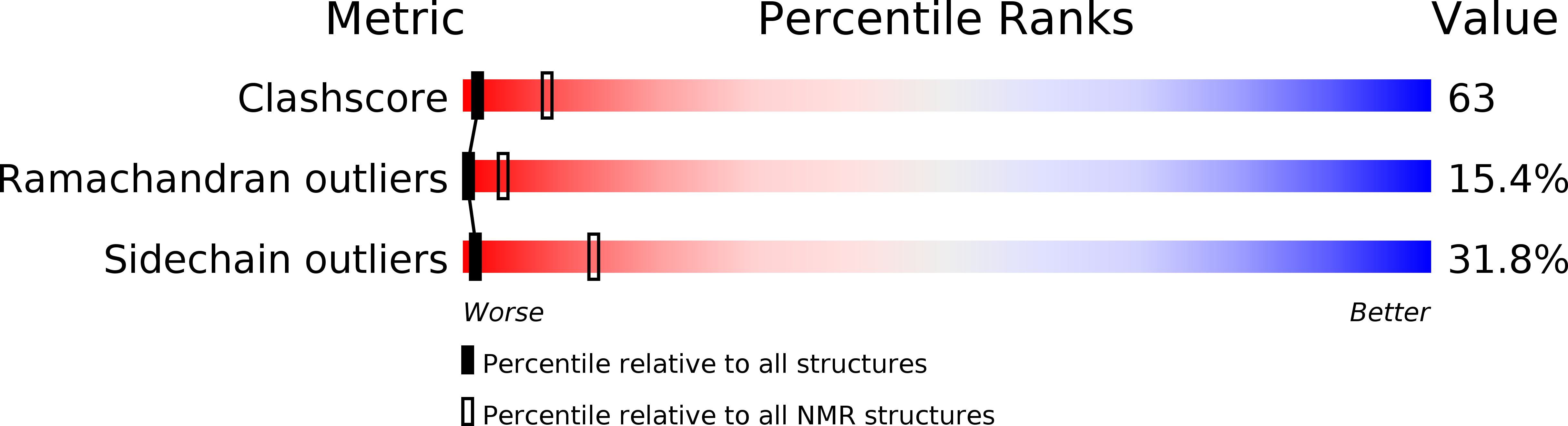Deposition Date
2002-05-16
Release Date
2002-05-22
Last Version Date
2024-05-08
Entry Detail
PDB ID:
1J5M
Keywords:
Title:
SOLUTION STRUCTURE OF THE SYNTHETIC 113CD_3 BETA_N DOMAIN OF LOBSTER METALLOTHIONEIN-1
Method Details:
Experimental Method:
Conformers Submitted:
1