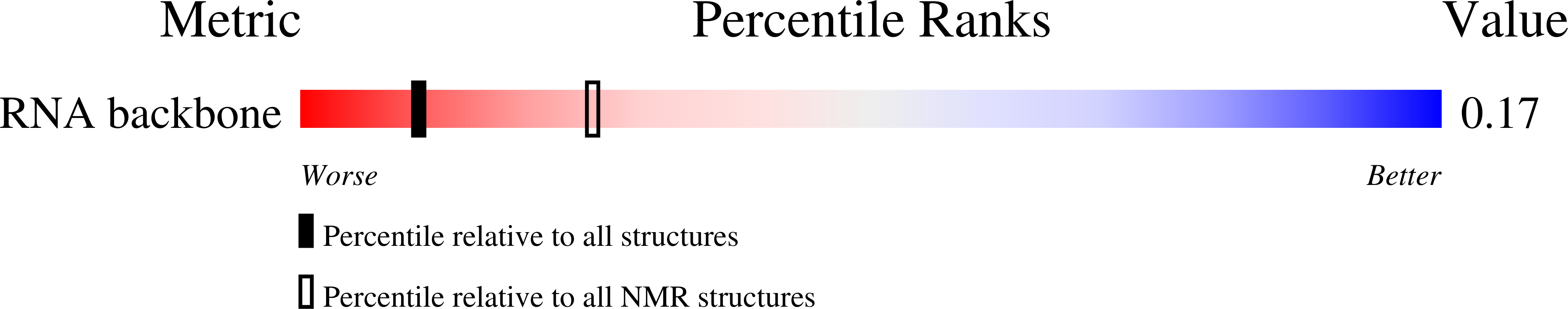
Deposition Date
2001-12-12
Release Date
2002-07-17
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1J4Y
Keywords:
Title:
Solution Structure of the Unmodified Anticodon Stem-loop from E. coli tRNA(Phe)
Biological Source:
Source Organism:
Method Details:
Experimental Method:
Conformers Submitted:
1