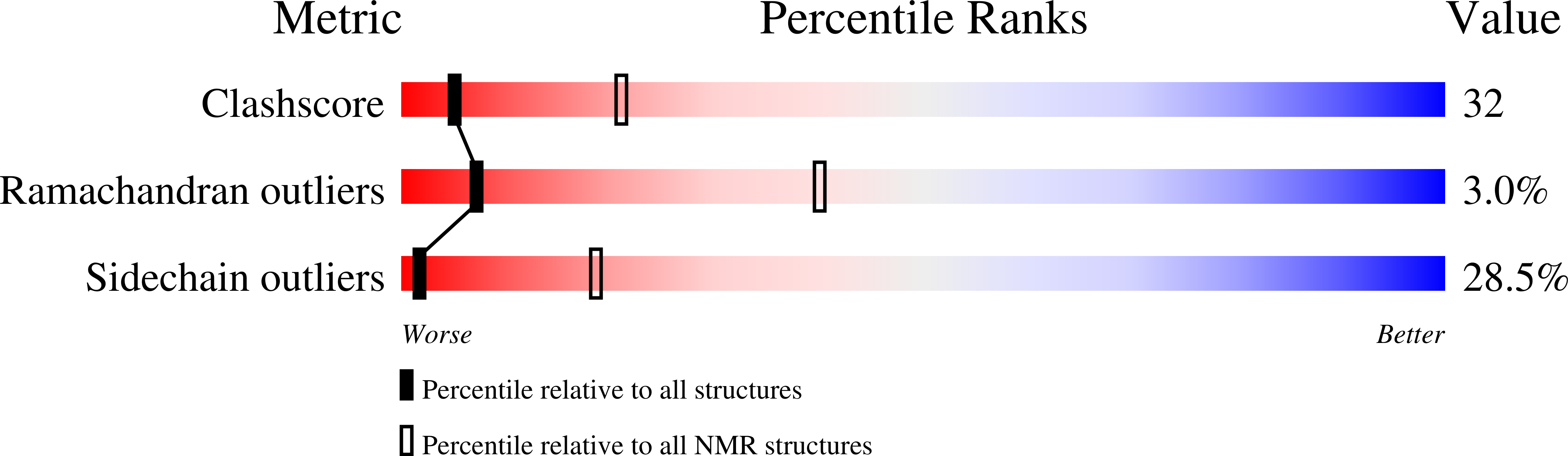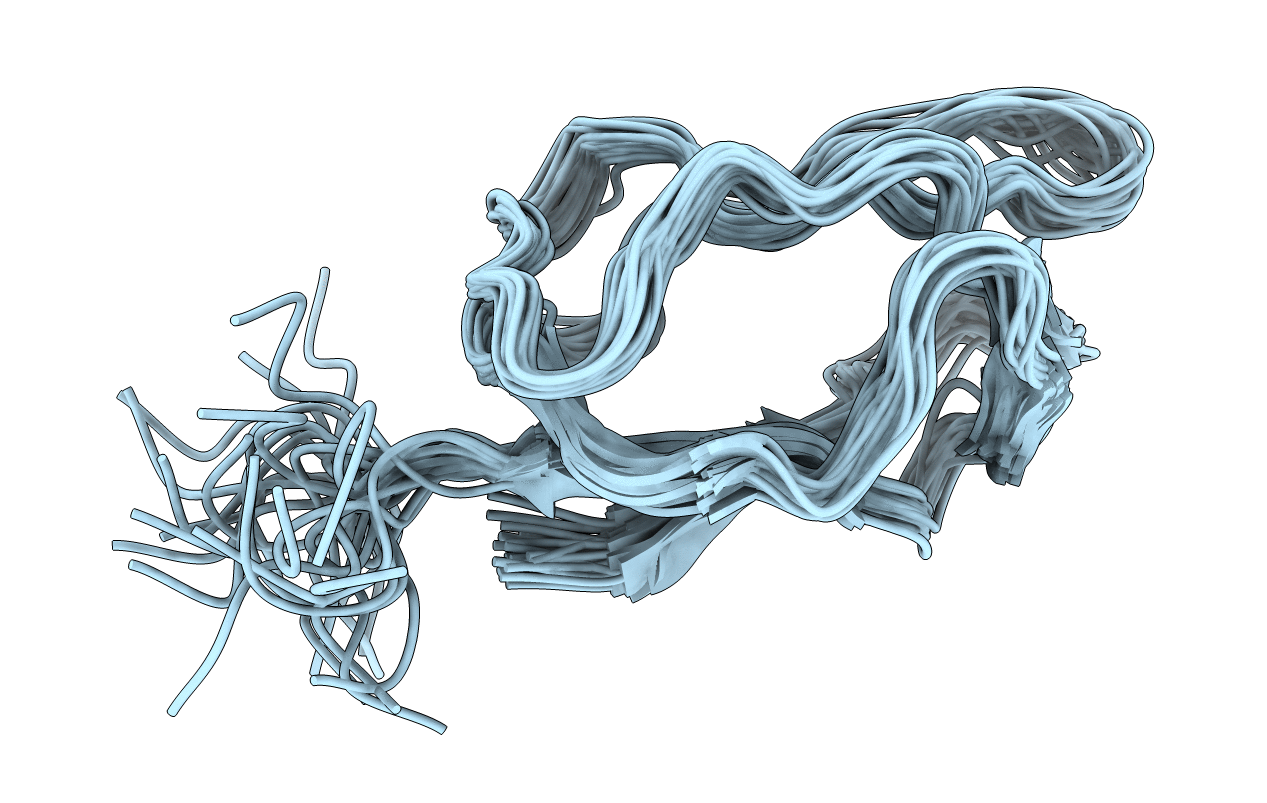
Deposition Date
1996-09-25
Release Date
1997-03-12
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1IYV
Keywords:
Title:
LIPOYL DOMAIN OF PYRUVATE DEHYDROGENASE COMPLEX, NMR, 29 STRUCTURES
Biological Source:
Source Organism(s):
Azotobacter vinelandii (Taxon ID: 354)
Method Details:
Experimental Method:
Conformers Submitted:
29